Organ Transplants: Changes in Allocation Policies for Donated Livers and Lungs
Fast Facts
For patients with severe organ failure, transplants can be lifesaving. Yet thousands remain on waiting lists in the U.S. to receive transplants, and more than 20 people die each day waiting for an organ.
In the past, donated livers and lungs were generally offered first to the sickest patients within a donation service area (a location with fixed boundaries).
But policies have changed. Now, these organs are typically offered first to the sickest patients who are a specified distance from the donor hospital—allowing sick patients to be eligible for organs that are donated across larger geographic areas.

Highlights
What GAO Found
The Organ Procurement and Transplantation Network (OPTN) develops allocation policies in the United States to determine which transplant candidates receive offers for organs, such as livers or lungs, that are donated from deceased donors. In July 2018, the Department of Health and Human Services (HHS), which oversees OPTN, directed it to change the liver allocation policy to be more consistent with federal regulations. The liver allocation policy changed in February 2020 from a system that, in general, offered donated livers first to the sickest candidates within the fixed boundaries of a donation service area or region to a system based on a candidate's level of illness and distance from the donor hospital. The current liver allocation policy offers livers first to the sickest candidates within 500 nautical miles of the donor hospital using a series of distance-based concentric circles, called acuity circles.
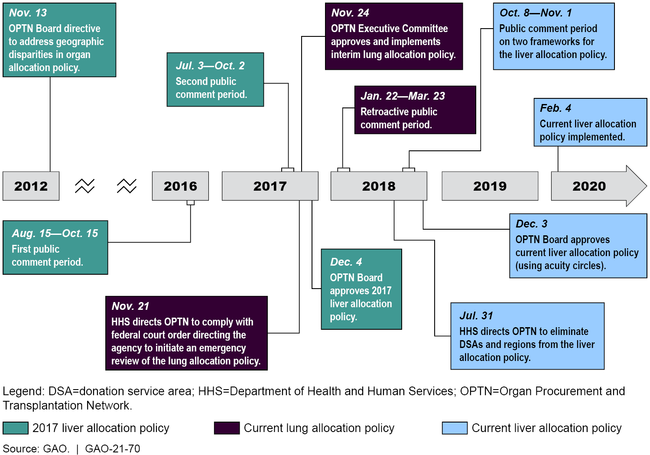
The processes used to develop the liver and lung allocation policies had various similarities and differences. For example, while the current liver allocation policy, the 2017 liver allocation policy, and the current lung allocation policy each had public comment periods, the length of these comment periods varied—25 days for the current liver allocation policy; two separate 62-day and 64-day periods for the 2017 liver allocation policy; and 61 days (retroactive) for the current lung allocation policy. In addition, the current lung allocation policy resulted in part from a federal district court order directing HHS to initiate emergency review of the policy. However, the 2017 liver allocation policy—that was approved but never implemented—resulted from a 2012 OPTN Board directive to reduce geographic disparities in organ allocation. HHS oversight of OPTN's processes were similar for all three allocation policies and included reviewing the proposed changes to the policies to ensure compliance with federal regulations, according to HHS officials.
Timeline of Selected Events Related to Three Organ Allocation Policies
Why GAO Did This Study
Organ transplantation is the leading form of treatment for patients with severe organ failure. OPTN, a nonprofit entity that was established in 1984 under the National Organ Transplant Act, manages the nation's organ allocation system. In 2019, 32,322 organs were transplanted from deceased donors in the United States. Nevertheless, as of July 2020, close to 110,000 individuals remained on waiting lists for donor organs. Previously, donated livers and lungs were generally offered first to the sickest candidates in donation service areas. However, livers and lungs are now generally offered first to the sickest candidates based on distance.
GAO was asked to review the changes to the liver and lung allocation policies. This report describes (1) changes to the liver allocation policy, and (2) similarities and differences in the processes OPTN used to change the liver and lung allocation policies, and federal oversight of these processes, among other things.
GAO reviewed documents, including those related to the current liver and lung allocation policies, and the 2017 liver allocation policy; interviewed HHS officials and OPTN members; reviewed the National Organ Transplant Act and its implementing regulations; and conducted a literature review of studies published from January 2017 through April 2020 in peer-reviewed and other publications.
HHS and the United Network for Organ Sharing (the contractor serving as OPTN) provided technical comments on a draft of this report, which GAO incorporated as appropriate.
For more information, contact James Cosgrove at (202) 512-7114 or cosgrovej@gao.gov.
