Biomedical Research: Improvements Needed to the Quality of Information About DOD and VA Contributions to Drug Development
Fast Facts
The Departments of Defense and Veterans Affairs' research on conditions such as spinal cord injuries and PTSD has contributed to thousands of patents for new drugs and treatments.
But these patents don't always disclose federal funding as required. For example, 18% of biomedical patents we reviewed disclosed DOD support but didn't include a correct DOD award number. This makes it hard to evaluate the impact of federal funding on innovation.
We recommended improving training for DOD staff to ensure awardees accurately disclose DOD support in patents. DOD and VA should also ensure the trials they fund are reported promptly on ClinicalTrials.gov.

Highlights
What GAO Found
The Departments of Defense (DOD) and Veterans Affairs (VA) fund biomedical research and development (R&D) that can lead to new drugs and medical devices. DOD and VA dedicated about $20 billion and $10 billion respectively to such R&D in fiscal years 2019 through 2023. Multiple DOD components fund biomedical R&D. The majority of it is performed by external entities, such as universities. VA's R&D is conducted by VA researchers at more than 100 VA medical centers.
DOD- and VA-funded research can lead to the patenting of new inventions. Among biomedical patents with application dates in fiscal years 2014 through 2023, GAO identified 1,146 patents owned by DOD and VA, as well as 3,078 biomedical patents owned by other entities disclosing DOD support. Only federal employees conduct VA-funded research, and VA generally owns the resulting patents. GAO found that 559 of the 3,078 patents did not disclose a correct DOD award number as required. DOD does not provide department-wide training on disclosure of agency support in patents to its personnel responsible for reviewing awardee disclosures. Without DOD personnel ensuring consistent and accurate awardee disclosure of DOD support in patents, the public and policymakers cannot measure the full extent of DOD's contributions to biomedical technologies, including drugs.
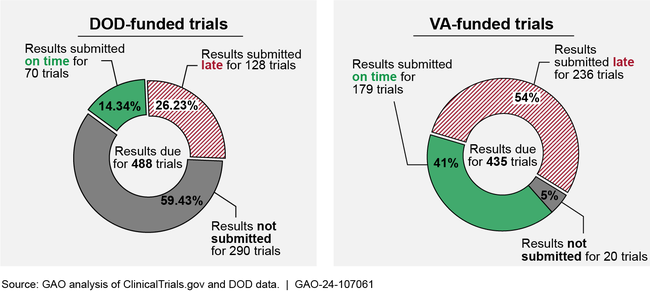
GAO found that, according to ClinicalTrials.gov data, among trials registered in fiscal years 2014 through 2023, results were submitted late or not at all for the majority of DOD- and VA-funded trials (see figure). DOD does not provide clear guidance for public reporting of clinical trial information. VA officials stated they saw a benefit to updating information resources for VA investigators and enhancing how investigators are notified of result submission deadlines, which could improve result reporting. Delays in result reporting hinder the transparency of federally funded clinical trials. Without complete and timely reporting, users of ClinicalTrials.gov—including patients, physicians, researchers, and the public—cannot access important scientific information about health outcomes and adverse events associated with those trials.
Figure: Result Reporting for Trials Funded by the Departments of Defense (DOD) and Veterans Affairs (VA) Registered on ClinicalTrials.gov in Fiscal Years 2014–2023
Why GAO Did This Study
DOD and VA prioritize developing biomedical technologies to treat diseases and conditions that affect military personnel and veterans, including brain and spinal cord injuries, infectious diseases, and post-traumatic stress disorder.
GAO was asked to review DOD and VA research contributions to drug development. This report examines (1) DOD and VA funding for biomedical R&D in fiscal years 2019 through 2023; (2) DOD and VA ownership of biomedical patents and the extent to which agency support is disclosed in other biomedical patents arising from research they fund; and (3) the extent to which information about DOD- and VA-funded clinical trials is publicly reported.
GAO reviewed relevant laws and agency documents; analyzed funding, patent, and clinical trial data; visited five DOD facilities and one VA medical center; and interviewed agency officials and 12 researchers who conducted agency-funded research. This report is a companion to GAO-23-105656, which examined National Institutes of Health research contributions to drug development.
Recommendations
GAO is making two recommendations to DOD and one to VA, including that DOD improve agency-wide training of personnel to better ensure accurate disclosure of DOD support in patents by awardees, and that DOD and VA both take steps to improve the completeness and timeliness of reporting on ClinicalTrials.gov for the trials they fund. DOD and VA agreed with the recommendations.
Recommendations for Executive Action
| Agency Affected | Recommendation | Status |
|---|---|---|
| Department of Defense | The Office of the Under Secretary of Defense for Acquisition and Sustainment, in collaboration with the Office of the Under Secretary of Defense for Research and Engineering, should enhance department-wide training for the DOD personnel who review information submitted by awardees to ensure that awardees disclose DOD support correctly in patents arising from DOD-funded research. (Recommendation 1) |
DOD concurred with the recommendation in its comments included in the report. When we confirm what actions the agency has taken in response to this recommendation, we will provide updated information.
|
| Department of Defense | The Office of the Assistant Secretary of Defense for Health Affairs should develop clear guidance requiring responsible parties of all trials funded by the Defense Health Program to report DOD as a funder when registering a trial on ClinicalTrials.gov, register the trial within 21 days of enrolling the first patient, and submit trial results within 1 year of the primary completion date to improve the quality and completeness of public information about DOD-funded clinical trials. (Recommendation 2) |
DOD concurred with the recommendation in its comments included in the report. When we confirm what actions the agency has taken in response to this recommendation, we will provide updated information.
|
| Department of Veterans Affairs | The Under Secretary of Health at VA should direct ORD to take steps to better ensure that VA investigators conducting ORD-funded clinical trials submit results to ClinicalTrials.gov within 1 year of the primary completion date. Such steps could include improving procedures for notifying VA investigators of the trial result submission deadlines and collecting and analyzing data to determine how to address factors causing delays. (Recommendation 3) |
VA concurred with the recommendation. In its written comments included in the report, VA stated ORD will focus on the subset of clinical trials that are not subject to legal result reporting requirements but are expected to adhere to VA's ethically based reporting requirements; will expend additional additional resources to collect and analyze data to determine what factors are contributing to delays in result reporting for these trials; and will develop and implement strategies to better ensure timely results reporting to ClinicalTrials.gov specifically for these trials. When we confirm what actions the agency has taken in response to this recommendation, we will provide updated information.
|
