Military Health Care: DOD Should Improve Its Process for Clinical Adverse Actions against Providers
Fast Facts
The Defense Health Agency investigates concerns about the quality and safety of care that individual health care providers deliver in DOD medical facilities. If DHA finds evidence for those concerns, it can prohibit providers from DOD facilities or limit the services they can provide.
DHA must also report the provider to a federal database that hospitals and others can use to screen providers. But DHA didn't always report them within the required 30-day timeframe. Also, it hasn't set timeliness standards for other steps in this process, such as legal reviews and appeals meetings.
We recommended addressing timeliness issues in this process.

Highlights
What GAO Found
The Defense Health Agency (DHA) uses its clinical adverse action process to investigate concerns about a health care provider's quality of care, and if warranted, to take action to limit or prohibit the care a provider is allowed to deliver. GAO reviewed 55 clinical adverse action cases at four selected military medical treatment facilities and found that they did not always adhere to certain requirements. For example, in more than one-third of the cases, the facilities did not adhere to the DHA requirement to establish a deadline for the investigation of a provider. GAO found that, while DHA monitors facilities' adherence by conducting an audit of each case and by monitoring the process, DHA's monitoring approach does not include information needed to assess adherence to many of the facility-level steps of the clinical adverse action process.
GAO also found that DHA did not always report providers within required time frames to the National Practitioner Data Bank. This database is an electronic repository administered by the federal government that is used by hospitals and others across the health care industry to obtain information on providers with histories of substandard care or misconduct. GAO found that while DHA reported all 14 of the providers from the four facilities in GAO's review who received a final clinical adverse action, DHA did not meet the 30-day reporting requirement for four providers.
Defense Health Agency Adherence to Requirements for Reporting 14 Final Clinical Adverse Actions to the National Practitioner Data Bank
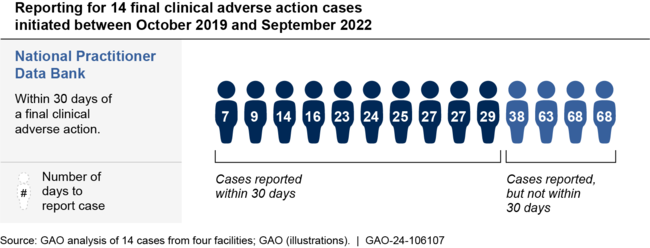
DHA's approach to monitoring its clinical adverse action process does not include information needed to assess adherence to certain requirements, such as whether DHA reports providers within required time frames. Further, DHA has not established timeliness requirements for many of the DHA-level steps in the process, such as legal reviews and appeal panel meetings. GAO found it took DHA almost one year on average to complete its steps for 14 cases that resulted in final clinical adverse actions. While DHA's procedures state that the purpose of the clinical adverse action process is to ensure timely resolution of issues and reporting, GAO found that DHA does not sufficiently monitor its timeliness. Such deficiencies could present risks to the quality and safety of care that military service members and their families receive in DOD facilities.
Why GAO Did This Study
Like all health care delivery settings, concerns may arise about the quality and safety of care delivered by individual health care providers in the Department of Defense's (DOD) military medical treatment facilities. DHA and its medical facilities share responsibility for investigating concerns and determining whether to take clinical adverse action against providers. DHA is also responsible for reporting any actions taken against providers to regulatory bodies for use among the health care industry.
Senate Report 117-39 accompanying the National Defense Authorization Act for Fiscal Year 2022 includes a provision for GAO to review DOD's clinical adverse action process. GAO's review examines adherence to DHA clinical adverse action requirements at four selected facilities and at the DHA-level. GAO reviewed documentation of 55 clinical adverse action cases initiated between October 2019 and September 2022 by four facilities, selected to obtain variation in location and the number of clinical adverse actions conducted. Additionally, GAO reviewed DHA procedures and interviewed DHA officials and facility staff.
Recommendations
GAO is making six recommendations, including for DHA to improve its monitoring approach and to establish timeliness requirements for steps in the clinical adverse action process. DOD concurred with all six recommendations.
Recommendations for Executive Action
| Agency Affected | Recommendation | Status |
|---|---|---|
| Defense Health Agency | The Director of DHA should modify its monitoring reports or audit tools to capture information needed to effectively assess adherence to certain requirements, such as notification to other health care entities of a provider's summary suspension and state licensing board reporting. (Recommendation 1) |
DOD agreed with this recommendation. We will update the status when DHA provides additional information about actions taken.
|
| Defense Health Agency |
Priority Rec.
The Director of DHA should strengthen its monitoring of MTFs' and DHA's timeliness in completing the steps in the clinical adverse action process. (Recommendation 2) |
DOD agreed with this recommendation. We will update the status when DHA provides additional information about actions taken.
|
| Defense Health Agency | The Director of DHA should clarify in the DHA procedures manual for clinical adverse actions that MTFs must summarily suspend providers at the initiation of all clinical adverse action cases. (Recommendation 3) |
DOD agreed with this recommendation. We will update the status when DHA provides additional information about actions taken.
|
| Defense Health Agency | The Director of DHA should clarify in the DHA procedures manual for clinical adverse actions the requirements for documenting the MTFs' final privileging authority decisions. This should include specifying that implementation of the privileging authority decision should be documented in a timely manner. (Recommendation 4) |
DOD agreed with this recommendation. We will update the status when DHA provides additional information about actions taken.
|
| Defense Health Agency | The Director of DHA should establish timeliness requirements for the DHA-level procedures in the clinical adverse action process, including the DHA audit, legal sufficiency review, clinical peer review, appeal panel meeting and recommendation, and final report authority decision. (Recommendation 5) |
DOD agreed with this recommendation. We will update the status when DHA provides additional information about actions taken.
|
| Office of the Assistant Secretary of Defense (Health Affairs) | The Assistant Secretary of Defense for Health Affairs should require DHA to report data on its adherence to clinical adverse action reporting requirements, such as the number of providers with reportable actions and the timeliness of reports, and should use this information to improve its oversight of DHA. (Recommendation 6) |
DOD agreed with this recommendation. We will update the status when DOD provides additional information about actions taken.
|
