Institutional Review Boards: Actions Needed to Improve Federal Oversight and Examine Effectiveness
Fast Facts
Institutional Review Boards (IRBs) assess the ethics and safety of research studies involving human subjects, such as behavioral studies or clinical trials for new drugs or medical devices.
Health and Human Services oversees about 2,300 U.S.-based IRBs through routine or for-cause inspections to assess if they are following federal laws when reviewing research. But few IRBs are inspected. For example, one HHS agency aims to do just 3-4 routine inspections each year. Also, HHS agencies haven't examined how many inspections are needed or if inspections could be changed to further reduce risks to human subjects.
Our recommendations address this.

Highlights
What GAO Found
Institutional review boards (IRB) are groups that review ethical and safety considerations for research involving human subjects, such as clinical trials.
General Institutional Review Board (IRB) Process
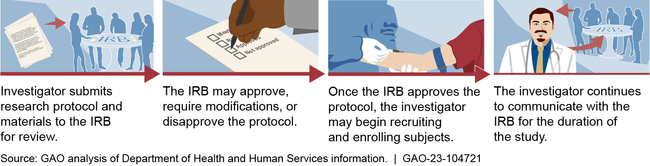
Most IRBs are based at universities, according to Department of Health and Human Services (HHS) data. University-based IRBs were also responsible for reviewing most research involving certain investigational drugs from calendar years 2012 through 2020, according to Food and Drug Administration (FDA) data. Some IRBs are independent, meaning they are not part of institutions that conduct or sponsor research. FDA data show these independent IRBs have reviewed an increasing share of investigational drug research: 25 percent of this research in 2012, and 48 percent in 2021. At the same time, the number of independent IRBs has decreased largely due to consolidation; this is, in part, related to private equity investment in IRBs.
FDA and HHS's Office for Human Research Protections (OHRP) oversee about 2,300 U.S.-based IRBs (operated by about 1,800 separate organizations, which may register and operate one or more IRB) through routine or for-cause inspections. These inspections assess whether IRBs follow federal regulations when reviewing research. FDA and OHRP consider several factors when selecting organizations for inspections, such as the volume of research reviewed. However, GAO found the agencies inspect relatively few IRBs. OHRP officials said they aim to conduct three to four routine inspections annually, while FDA conducted an average of 133 inspections annually between fiscal years 2010 and 2021. Neither agency has conducted a risk-based assessment of their IRB inspection program to help ensure they inspect enough IRBs annually and to optimize their responsibilities in protecting human subjects. Such an approach would be consistent with federal risk management principles.
While the agencies oversee IRBs to determine their adherence to regulations, OHRP and FDA have not assessed to what extent IRB reviews are effective in protecting human subjects. This is because the agencies have not determined the best approaches for doing so. Evaluating effectiveness is challenging in part due to an absence of validated measures and because IRBs are only one part of the framework of stakeholders responsible for protecting human subjects. Convening stakeholders to identify approaches for evaluating IRB effectiveness would be consistent with OHRP and FDA responsibilities and change management practices, and would help provide assurance that IRBs are successful in protecting human subjects.
Why GAO Did This Study
IRBs review research studies involving human subjects to ensure that risks to subjects are minimized and participants have sufficient information to consent to participate. In the past, IRBs were based at research institutions, such as academic centers. Over time, independent IRBs have played a more prominent role in reviewing research on human subjects. Some policymakers and others have raised questions about the increased use of independent IRBs and the effects on protecting human subjects.
GAO was asked to examine independent IRBs, processes used to protect human subjects, and standards of IRB quality, among other things. This report describes the composition of the IRB market and examines OHRP and FDA oversight of IRBs, among other objectives.
GAO reviewed federal laws and regulations and articles published between 2010 and June 2021; analyzed IRB registration, drug application, and inspection data; and interviewed FDA and OHRP officials, experts and stakeholders, and 11 IRBs selected for variation in type, size, and other factors.
Recommendations
GAO is making four recommendations, including that HHS and FDA conduct annual risk assessments to determine if the agencies are routinely inspecting an adequate number of IRBs and to optimize the use of inspections in the oversight of IRBs and protection of research participants, and examine and implement approaches for measuring IRB effectiveness. HHS concurred with the recommendations.
Recommendations for Executive Action
| Agency Affected | Recommendation | Status |
|---|---|---|
| Department of Health and Human Services | 1. The Assistant Secretary for Health should ensure that OHRP takes steps to ensure the accuracy of protocol data collected in OHRP's IRB registry. This could include updating instructions to IRBs and examining data accuracy for a sample of IRBs. (Recommendation 1.) |
In August 2023, HHS reported that OHRP was taking steps to address this recommendation. First, HHS noted OHRP was working to modify its IRB registration system to prompt IRBs applying for, renewing, or updating their OHRP registration to provide accurate information about the number of protocols they review. Additionally, HHS reported OHRP planned to evaluate its IRB registration instructions as part of the next Paperwork Reduction Act Information Collection Request renewal; the current IRB registration form is approved through June 30, 2025. As of March 2024, we had not yet received updated information from HHS and will update the status when we do.
|
| Department of Health and Human Services | 2. The Assistant Secretary for Health should ensure that OHRP conducts an annual risk assessment to determine whether the agency is conducting an adequate number of routine IRB inspections and to optimize the use of IRB inspections in the oversight of IRBs and protection of research participants. (Recommendation 2) |
In August 2023, HHS reported that OHRP was taking steps to address this recommendation. HHS noted OHRP was considering options for conducting the annual risk assessment, and had or planned to discuss approaches with FDA and other federal entities that support or conduct human subjects. As of March 2024, we had not yet received updated information from HHS and will update the status when we do.
|
| Food and Drug Administration | 3. The Commissioner of the Food and Drug Administration should conduct an annual risk assessment to determine whether the agency is conducting an adequate number of routine IRB inspections and to optimize the use of IRB inspections in the oversight of IRBs and protection of research participants. (Recommendation 3) |
In August 2023, HHS reported that FDA was taking steps to address this recommendation. HHS reported FDA planned to take steps to assess the number of annual IRB inspections it conducts by March 2024, to account for inspections conducted in fiscal year 2023 and 2024. As of March 2024, we had not yet received updated information from HHS and will update the status when we do.
|
| Department of Health and Human Services | 4. The Secretary of Health and Human Services should ensure that OHRP and FDA convene stakeholders to examine approaches for measuring IRB effectiveness in protecting human subjects, and implement the approaches as appropriate. These could include effectiveness measures; peer audits of IRB meetings and decisions; mock protocols; surveys of IRB members, investigators, and human research participants; or other approaches. (Recommendation 4) |
In August 2023, HHS reported that OHRP and FDA have taken steps to address this recommendation. HHS noted that OHRP and FDA had begun identifying and convening stakeholders to examine approaches for measuring IRB effectiveness in protecting human subjects. For example, HHS asked the HHS Secretary's Advisory Committee on Human Research Protections (SACHRP) to consider such approaches; in response, SACHRP issued its recommendation on October 19, 2023. According to HHS, OHRP and FDA would consider those recommendations, once made. OHRP and FDA also met or planned to meet with other stakeholders (e.g., the Consortium to Advance Effective Research Ethics Oversight and attendees of the Public Responsibility in Medicine & Research's December 2023 annual conference), to discuss and obtain input on efforts and metrics to measure IRB effectiveness. As of March 2024, we had not yet received updated information from HHS. We will continue to update the status of this recommendation when we receive additional information on actions OHRP or FDA have completed to examine approaches for measuring IRB effectiveness, and in taking any steps to implement such approaches.
|
