COVID-19: Agencies Are Taking Steps to Improve Future Use of Defense Production Act Authorities
Fast Facts
The Defense Production Act allows federal agencies to require companies to prioritize government contracts for medical supplies to address national emergencies, like COVID-19. Federal agencies have used DPA authorities to expand production of COVID-related medical supplies.
Federal agencies faced challenges using DPA authorities. For example, the Department of Health and Human Services had limited experience using DPA pre-pandemic. To help, HHS established offices to manage DPA activities and production expansion projects. But, it has not developed a plan to use DPA to address future medical supply needs as we previously recommended.
Highlights
What GAO Found
Federal agencies used the Defense Production Act (DPA) and other actions over 100 times to help address COVID-19 medical supply needs through September 2021. Agencies used DPA authorities to 1) prioritize contracts so those orders can get preference over others, (2) fund projects to expand domestic production of supplies, and (3) enter into partnerships with private companies (see figure).
Defense Production Act and Other Actions, March 2020-September 2021
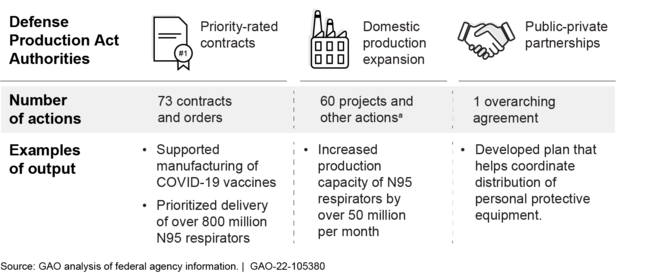
aOther actions refer to production expansion projects not executed under DPA Title III authority.
Representatives from companies that received DPA awards generally stated that the use of the DPA gave them timely access to raw materials and supplies and helped them expand production faster than they could have on their own.
GAO previously reported that federal agencies faced challenges using DPA authorities. Agencies have taken some steps to address these obstacles as well as one of two recommendations GAO made.
- The Department of Health and Human Services (HHS), which had limited DPA experience prior to COVID-19, reported establishing offices to review DPA priority rating requests and to manage industrial base expansion efforts. However, it has not developed a plan for using DPA and other actions to address future medical supply needs as GAO recommended.
- The Department of Defense, which has been providing significant contracting support to HHS, documented procedures to facilitate the timely transfer of funds between the two agencies and established a permanent office in October 2020 to support interagency needs.
- The Office of Management and Budget established a web page to collect and publish data on DPA priority ratings, which addressed a prior GAO recommendation concerning transparent reporting of these DPA actions.
Additional DPA and other actions are expected through 2025 as agencies use $10 billion appropriated in the American Rescue Plan Act for medical supply investments and implement a September 2021 national strategy to strengthen the domestic medical industrial base. GAO plans to monitor agencies' efforts.
Why GAO Did This Study
COVID-19 put the U.S. health care system under severe strain, affecting federal agencies' ability to buy and maintain critical medical supplies to help treat patients and protect health care workers. The federal government's COVID-19 response included a significant use of DPA authorities, as well as other actions focused on expanding domestic production of medical supplies to help stabilize the medical supply chain. The CARES Act and other supplemental appropriations provided at least $11 billion for DPA purchases and other actions related to COVID-19 or other public health emergencies through September 2025.
The CARES Act includes a provision for GAO to monitor funds provided for the COVID-19 pandemic. This report summarizes federal agencies' use of the DPA to respond to COVID-19 and industry perspectives, as well as implementation challenges and steps agencies have taken to address these challenges. This report summarizes information contained in reports issued in June, September, and November 2020, and January, March, April and July 2021, and provides data updates through September 2021.
Recommendations
In November 2020, GAO recommended that HHS identify how the DPA and other actions will be used to increase production of domestic medical supplies and reduce U.S. reliance on foreign sources. HHS agreed but has not yet determined what specific actions it will take.
