Vaccine Development: Capabilities and Challenges for Addressing Infectious Diseases
Fast Facts
The CARES Act requires GAO to look at the government's response to the COVID-19 pandemic, including vaccine development. Vaccines prepare the body to respond quickly to an infection and play a key role in keeping people and global communities healthy.
Overall, vaccine development is still difficult, complex, and costly. But we identified 16 innovative technologies and approaches that may enhance the nation's ability to respond to high-priority infectious diseases. Some innovative technologies—like reverse vaccinology that uses computers to analyze the genetics of a pathogen—helped researchers develop COVID-19 vaccines quickly and effectively.
Highlights
What GAO Found
Vaccines protect people from disease by preparing the body to respond to an infection. Vaccinations are a key part of individual and community health, but vaccine development remains complex and costly. Innovative technologies and approaches, such as those identified in this report, may enhance the nation’s ability to respond to infectious disease. For example, reverse vaccinology and next-generation platforms—combined with existing research—helped researchers develop some COVID-19 vaccines more quickly and effectively.
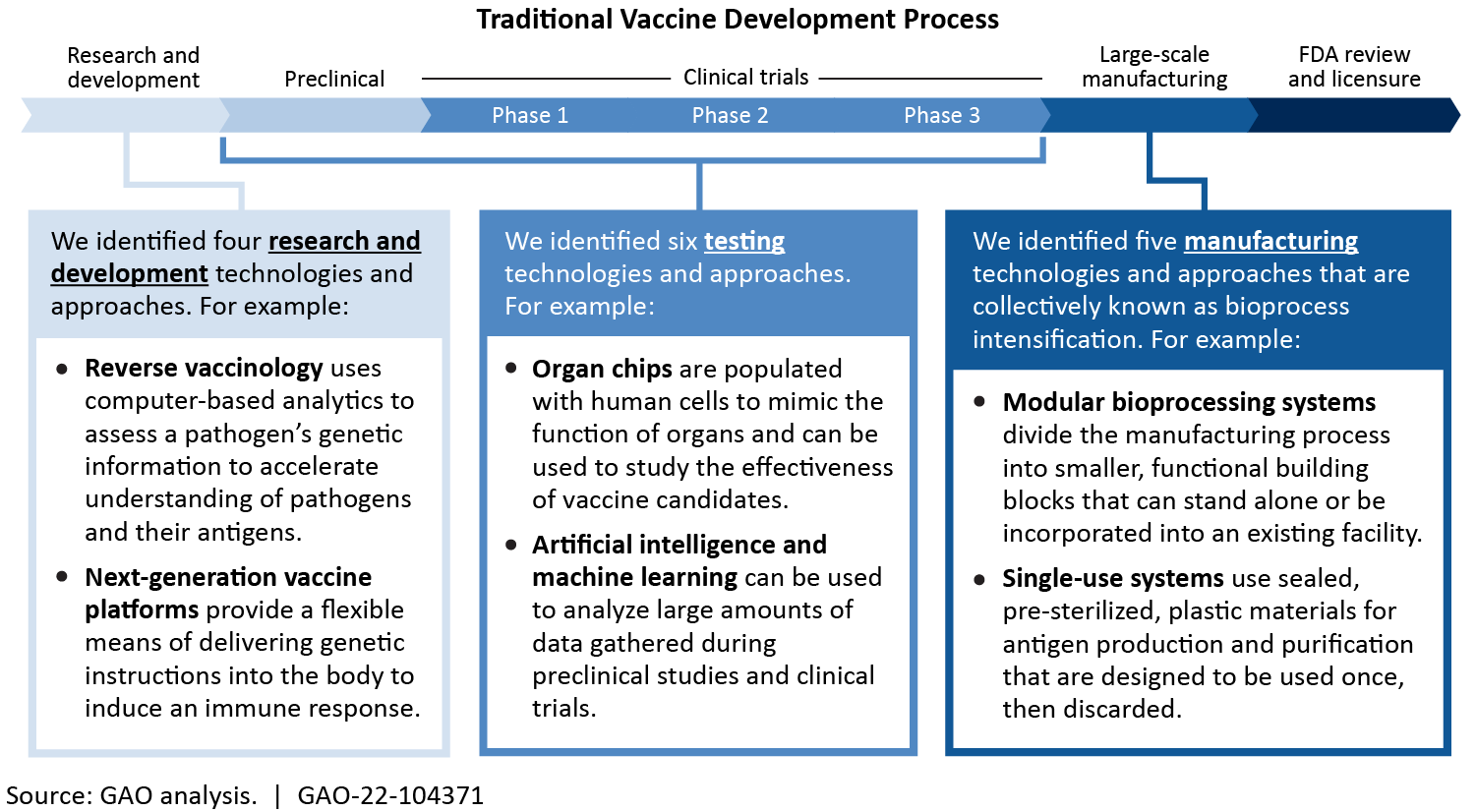
However, key challenges may hinder the adoption of these innovative technologies and approaches. Some promising technologies face issues and challenges such as inherent technical limitations and high cost. For example, organ chips may facilitate testing, but they are not yet able to replicate many of the complex functions of the human immune system. Similarly, single-use systems may increase the flexibility of vaccine manufacturing facilities, but may require extensive testing to ensure that they do not negatively affect the resulting vaccine. Further, economic challenges may hinder vaccine development. Experts attribute underinvestment in vaccines to market failures (i.e. market interactions that fall short of what would have been socially beneficial). For example, vaccines benefit those who are vaccinated, and, to some degree, those who are not. This additional benefit is not captured in the price, which reduces return on vaccine investment.
GAO identified 9 policy options that may help address challenges hindering the adoption of vaccine development technologies and approaches or economic challenges. These policy options involve possible new actions by policymakers, who may include Congress, federal agencies, state and local governments, academic and research institutions, and industry. See below for details for some of the policy options and relevant opportunities and considerations.
Selected Policy Options to Address Challenges in Vaccine Development
| Opportunities | Considerations | |
|
Prioritize infectious disease pathogens (report page 21) Policymakers could collaborate across sectors (e.g., government, academia, researchers, industry, and nonprofit organizations) to prioritize infectious disease pathogens with pandemic potential for vaccine R&D. For example, policymakers could develop a working group to prioritize pathogens with pandemic potential and work more closely with international organizations to prioritize vaccine development as well as develop monoclonal antibodies. |
|
|
|
Improve preparedness (report page 21) Policymakers could provide support for public-private partnerships to strategically address potential pandemic pathogens identified as priorities. These partnerships could, for example, develop and test vaccine candidates that may provide protection from pathogens with pandemic potential. |
|
|
|
Further support development of data standards (report page 32) Policymakers could further support coordinated efforts to obtain the views of all stakeholders and to develop standards for health data and their use in clinical trials. |
|
|
|
Improve preparedness (report page 41) Policymakers could provide support for public/private partnerships to strategically develop manufacturing capacity to respond to surge requirements. To maintain this capacity, partnerships could manufacture prototype vaccine candidates against high-priority pathogens. |
|
|
|
Evaluate factors that inhibit vaccine investment and mechanisms to increase it (report page 54) Policymakers could collaborate across sectors, such as government, academia, and industry, to conduct a systematic evaluation of factors that inhibit developers from investing in new vaccines. |
|
|
Source: GAO. | GAO-22-104371
Why GAO Did This Study
The CARES Act included a provision for GAO to report on its ongoing monitoring and oversight efforts related to the COVID-19 pandemic. This report discusses technologies, approaches, and associated challenges for vaccine (1) research and development, (2) testing, and (3) manufacturing, as well as (4) the economic factors that affect vaccine investment.
GAO conducted literature searches including scholarly articles and government reports relevant to these four areas. GAO interviewed stakeholders and experts with a diverse set of perspectives on the science, administration, and economics of vaccine development. GAO also convened a 3-day meeting of 22 experts with expertise in at least one area related to our four objectives with assistance from the National Academies of Sciences, Engineering, and Medicine. GAO received technical comments on a draft of this report from 1 federal agency and 9 participants at its expert meeting, which it incorporated as appropriate.
GAO is identifying policy options in this report.
For more information, contact Karen L. Howard at (202) 512-6888 or howardk@gao.gov.
