COVID-19: Sustained Federal Action Is Crucial as Pandemic Enters Its Second Year
Fast Facts
In our 6th comprehensive report on the federal response to the pandemic, we identified multiple ways agencies can improve response efforts. For example, we recommended improvements in federal data to provide a clear picture of whether COVID-19 vaccines are being distributed equitably to communities of color, which are disproportionately affected by the virus. We also recommended that federal agencies establish controls to combat potential fraud.
As of January 2021, we had made 44 recommendations to agencies; 6 have been implemented. This report makes 28 new recommendations. Agencies should swiftly take action to address all recommendations.
Highlights
What GAO Found
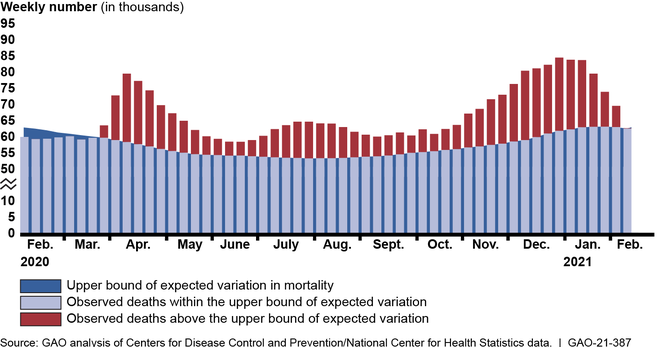
More than a year after the U.S. declared COVID-19 a public health emergency, the pandemic continues to result in catastrophic loss of life and substantial damage to the global economy, stability, and security. According to data from the Centers for Disease Control and Prevention’s (CDC) National Center for Health Statistics, about 520,000 more deaths occurred from all causes (COVID-19 and other causes) than would be normally expected from February 2020 through mid-February 2021, highlighting the effect of the pandemic on U.S. mortality (see figure). The pandemic also continues to cause economic challenges, particularly for the labor market. As of February 2021, there were about 10 million unemployed individuals, compared to nearly 5.8 million at the beginning of 2020.
Higher-Than-Expected Weekly Mortality in the U.S., February 2020 through Mid-February 2021
In the past year, GAO has made 44 recommendations for agency actions, 6 of which have been implemented. Since taking office, the new administration has taken some action consistent with GAO’s recommendations, such as issuing theNational Strategy for the COVID-19 Response and Pandemic Preparednessand issuing executive orders calling for the development of a pandemic supply chain resilience strategy and providing emergency economic relief.GAO will continue to monitor the administration’s actions toward addressing GAO’s recommendations in future reporting.
In this report GAO is making 28 new recommendations in the areas of public health, the economy, and program integrity. Implementing these 28 recommendations, as well as 38 of GAO’s 44 prior recommendations that have not been fully implemented from CARES Act reports issued since June 2020, would improve the ongoing federal response to COVID-19.
GAO’s new recommendations are discussed below.
Hospital and Pharmacy Perspectives on COVID-19 Vaccine Administration and Medical Supply Availability
In February 2021, GAO surveyed hospitals and interviewed large retail pharmacy chains and an association of independent pharmacies to gain their perspectives on vaccine administration and medical supply availability. Providers expressed concerns about COVID-19 vaccine availability and limitations in the availability of certain key medical supplies for administering the vaccines—notably syringes and needles. For example, representatives from one retail pharmacy chain stated that the chain has the capacity to administer 25 million doses per month at 9,900 locations, but the chain’s initial allocation of vaccines from the federal government was expected to be only 230,000 doses at 250 locations. Several retail pharmacy chain representatives also indicated that limited vaccine availability has led to uncertainty regarding the amount of vaccines their pharmacies can expect to receive each week. The new administration has taken steps to increase certainty and vaccine availability. For example, the White House announced at the end of January 2021 that the federal government would begin notifying states earlier about availability and shipments of vaccines, to give greater certainty for planning vaccination efforts.
Of the 146 surveyed hospitals that plan to or have begun administering COVID-19 vaccines, 40 hospitals reported at the time of GAO’s survey being greatly concerned about having a sufficient quantity of syringes in the next 30 days for vaccine administration following the survey, and 43 hospitals were greatly concerned about having a sufficient quantity of needles. Additionally, shortages of personal protective equipment (PPE) and COVID-19 testing supplies also remain a challenge for some providers. GAO and other entities have documented persistent and evolving supply chain challenges throughout the pandemic, such as shortages of key supplies used for COVID-19 testing. GAO will continue to examine the medical supply chain, including the role of the Strategic National Stockpile, in future reporting, including actions to respond to GAO’s previous recommendations.
Emergency Use Authorizations
Emergency use authorizations (EUA)—which allow for the temporary use of unapproved medical products—have been instrumental in increasing needed supply of certain devices, such as PPE, during the COVID-19 pandemic response (see figure). However, there have been instances of inconsistencies between EUAs issued by the Food and Drug Administration (FDA) and device guidance from CDC and the Department of Labor (DOL), which led to confusion and hesitancy among providers about using such devices, according to provider associations. GAO recommends that FDA, CDC, and DOL work together to develop a process for sharing information to facilitate decision-making and guidance consistency related to devices with EUAs. The Department of Health and Human Services (HHS)—which includes FDA and CDC—and DOL agreed with this recommendation.
Examples of Medical Devices Other Than Tests with Emergency Use Authorizations for COVID-19
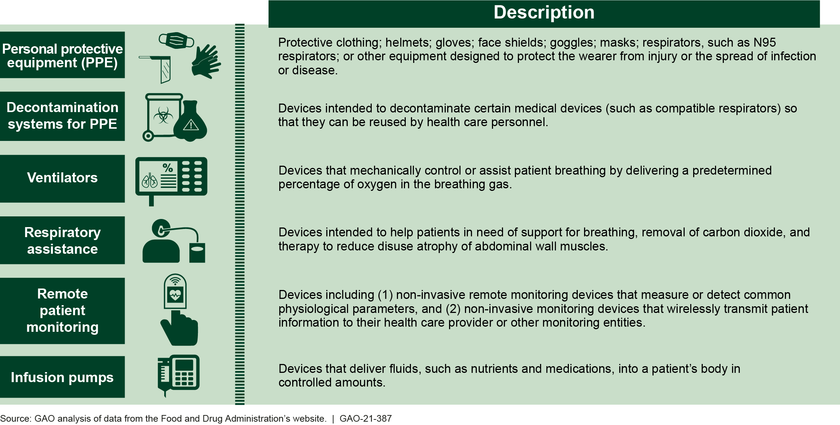
In addition, stakeholders—including associations representing manufacturers, distributors, and users of authorized devices, such as health care providers—raised concerns about what will happen to devices with EUAs after the declarations permitting their use for COVID-19 end. HHS has indicated that it intends to develop draft guidance for a transition plan for medical devices distributed under EUAs for COVID-19 by the end of fiscal year 2021. A plan for devices with EUAs that specifies a reasonable timeline and process for transitioning away from their use, taking into account stakeholder input, would help ensure a smooth transition. As HHS develops a transition plan for devices with EUAs, GAO recommends that the agency specify a reasonable timeline and process for transitioning authorized devices to clearance, approval, or appropriate disposition that takes into account input from stakeholders. HHS agreed with this recommendation.
COVID-19 Data for Health Care Indicators
Since June 2020, GAO has identified concerns with federal COVID-19 data and underscored that in the midst of a nationwide public health emergency, clear and consistent communication between the federal government and the public is critical given that effective response requires the public’s participation. As part of its efforts to communicate with the public and stakeholders about the pandemic, several experts suggested that the federal government should improve the accessibility of its COVID-19 data by making these data available from a central location on the internet. HHS publishes its data on COVID-19 health indicators across several websites. However, the data it makes publicly available are not all located on, or available from website links on, one online location. As a result, the public, including stakeholders, may not be able to fully understand the extent of the pandemic and use the data to best inform their decision-making.
To make the data more easily accessible, GAO recommends that HHS make its different sources of publicly available COVID-19 data accessible from a centralized location on the internet. HHS neither agreed nor disagreed with this recommendation, but agreed that COVID-19 data should be made accessible to support communication with the public about the pandemic.
COVID-19 Health Disparities
GAO previously reported that communities of color have been disproportionately affected by the pandemic. According to HHS, as of February 8, 2021, data collected from states and jurisdictions on COVID-19 vaccine recipients were missing data on race and ethnicity for almost half of recipients. Without complete information on the race and ethnicity of those vaccinated, HHS may have difficulty determining whether vaccines are distributed equitably to communities of color. GAO recommends that HHS take steps to ensure the complete reporting of race and ethnicity information for recipients of COVID-19 vaccinations. HHS neither agreed nor disagreed with this recommendation.
HHS’s July 2020 COVID-19 Response Health Equity Strategy has a goal to reduce health disparities by using data-driven approaches to attain the highest level of health possible for all individuals, including communities of color. However, the strategy lacks important elements of an effective national strategy. For example, HHS’s strategy does not provide specific actions that the agency will take to determine whether or where it needs to increase access to testing for populations at increased risk for COVID-19—an essential first step before taking steps to increase testing access. GAO recommends that HHS incorporate key elements of a national strategy to implement the agency’s COVID-19 Response Health Equity Strategy, including determining how intermediate outcomesshould be prioritized. HHS agreed with this recommendation.
Nursing Homes
Collecting detailed information on vaccinations for nursing home populations is important for tracking and transparency, particularly because nursing homes have been an epicenter of the COVID-19 pandemic and HHS has recommended priority vaccinations for this group. HHS established a pharmacy partnership program for vaccinating staff and residents of long-term care facilities, and publicly reports the number of vaccination doses, by state, provided to residents and staff of all long-term care facilities participating in the program. However, HHS does not report data showing vaccination rates specifically for nursing homes and does not collect or report data for nursing homes not participating in the program. To improve the monitoring and transparency of nursing home vaccination efforts, GAO recommends that HHS collect data specific to COVID-19 vaccination rates in nursing homes and make these data publicly available. HHS neither agreed nor disagreed with this recommendation.
In addition, as of January 2021, HHS had not specified whether nursing homes would be required to offer COVID-19 vaccinations as they have with other vaccines and how these vaccinations would be incorporated into the agency’s nursing home quality strategy. Data on COVID-19 vaccinations in nursing homes will also be important for HHS’s ongoing efforts to monitor nursing home quality. GAO recommends that HHS require nursing homes to offer COVID-19 vaccinations toresidents and staffand design and implement associated quality measures. HHS neither agreed nor disagreed with this recommendation.
Veterans Health Care
According to the Department of Veterans Affairs (VA), many veterans enrolled in VA’s health care system are at a higher risk of infection or severe disease from COVID-19 due to their age or underlying health conditions. GAO identified several areas where VA can improve its vaccination efforts:
- VA does not have metrics related to staff and veterans who do not show (no-shows) for their vaccination appointments. Without data on no-shows, VA may be at risk for not being able to determine the extent to which staff and veterans are not showing up for appointments for their second vaccinations, and may miss opportunities to better target outreach to individuals not showing up for appointments.
- VA lacks targets for when it will move from one vaccination phase to another or within one phase for when the agency will move from one group of veterans to another, making it difficult for the department to assess progress.
- VA is utilizing a phased vaccine rollout; however, VA’s current metrics do not capture vaccine data by phases. As a result, VA is not able to determine which facilities may be at an earlier phase than others and direct resources or assistance to those facilities.
GAO recommends that VA (1) collect data on the number of staff and veterans who do not show up for a vaccination appointment to better monitor for completion of the second dose of the vaccine; (2) develop preliminary vaccination targets for when it will move from one vaccination phase to another; or within one phase, from one group of veterans to another; and (3) develop metrics to assess the number of vaccines administered by vaccine rollout phase to better assess progress and make any necessary adjustments. VA agreed with the first and third recommendations and agreed in principle with the second recommendation.
Nutrition Assistance
The U.S. Department of Agriculture (USDA) administers a number of federal nutrition assistance programs to vulnerable populations. Recent legislative and executive actions made several changes to these programs as the negative economic effects of the COVID-19 pandemic have continued. However, until recently, USDA had released minimal data about participation in these programs during the pandemic, and when the department released data in late January 2021, it did not publicly share sufficient information about data quality. In August 2020, USDA announced it had identified significant issues with the quality of state-reported data on two programs. As it worked to identify the root causes of the issues, USDA opted not to release participation data for any of its other nutrition assistance programs from July 2020 until late January 2021. When USDA released the data, the department did not explain how it resolved the data quality issues it previously disclosed, nor did it share necessary context to help stakeholders and the public understand and interpret the data.
As a result, stakeholders and the public lack sufficient information and appropriate context to interpret key program data and understand the effects of the pandemic on the programs. GAO recommends that USDA (1) provide sufficient context to help stakeholders and the public understand and interpret data on federal nutrition assistance programs during the pandemic and (2) disclose potential sources of error that may affect data quality during the pandemic, such as manual processing. USDA generally agreed with these recommendations.
Disaster Relief Fund and Assistance to Tribal Governments
Available data from HHS indicate that tribes are among communities of color bearing a disproportionate burden of COVID-19 positive tests, cases, hospitalizations, and deaths. The Federal Emergency Management Agency (FEMA), within the Department of Homeland Security (DHS), plays a key role in the ongoing COVID-19 pandemic response effort, including using the Disaster Relief Fund to provide Public Assistance grants to reimburse tribal governments, among others, for pandemic costs, such as testing supplies, PPE, and vaccine distribution.
Several tribal organizations reported challenges related to completing administrative requirements to request and receive Public Assistance. For example, two tribal officials told GAO that when requesting technical assistance from FEMA to help with disaster activities such as developing a Public Assistance Administrative Plan, FEMA did not have staff to assist. FEMA’s initial assessment report of its response to the pandemic noted challenges and recommended that FEMA develop a tribal nation engagement strategy that includes providing the resources and personnel throughout each region required to support program delivery for all tribal nations. However, as of March 2021, FEMA had not developed this strategy.
GAO recommends that FEMA provide timely and consistent technical assistance to support tribal governments’ efforts to request and receive Public Assistance as direct recipients, including providing additional personnel, if necessary, to ensure that tribal nations are ableto effectively respondto COVID-19. DHS agreed with this recommendation.
FEMA’s 2019 Tribal Consultation Policy specifies the process for consulting with tribes throughout the four phases that guide the agency in how to conduct regular and meaningful collaboration with tribes (see figure). However, GAO found that FEMA did not follow the tribal consultation process while developing an interim policy detailing eligible items for reimbursement under the Public Assistance program. If tribes had been formally consulted earlier in the process, they could have been in a better position to provide meaningful input to FEMA on how its policy might impact tribes. Further, there may have been less confusion on which items were considered eligible for reimbursement during the early months of the pandemic, and tribes could have made more informed decisions. GAO recommends that FEMA adhere to the agency’s protocols listed in the updated 2019 Tribal Consultation Policy by obtaining tribal input via the four phases of the tribal consultation process when developing new policies and procedures related to COVID-19 assistance. DHS agreed with this recommendation.
Overview of FEMA’s Tribal Consultation Policy Process

K-12 Education
The Department of Education (Education) has taken steps to track state and school district spending of certain COVID-19 relief funds, but the data give an incomplete picture of the status of funds and understate the rate at which funds are being used. According to data collected by Education, as of February 28, 2021, states and territories have spent about $6.1 billion of the approximately $75 billion appropriated through the Education Stabilization Fund for states’ and territories’ education needs. However, federal spending data alone provide an incomplete picture of states’ and school districts’ spending, as there are several factors that influence the rate at which funds appear to be spent. For example, there is often a significant gap between when a district “uses” the funds (i.e., orders, contracts for, installs, and pays for goods or services, such as information technology equipment) and when those funds are reported as “spent” in state and federal reporting systems, as is common in federal grants management processes.
According to Education officials, states award applicable funds to school districts so that the school districts can obligate those funds for specific purposes. The state does not transfer funds to the district until the district requests payment for services or deliverables received. Education officials do not consider the funds spent until the state requests payment for expenses. Given this gap between when a district uses funds and funds are recorded as spent, absent information on obligations, policymakers will not have complete information on how these funds are being used to address the pandemic-related education needs of America’s schoolchildren. GAO recommends that Education regularly collect and publicly report information on school districts’ financial commitments (obligations), as well as outlays (expenditures) in order to more completely reflect the status of their use of federal COVID-19 relief funds. For example, Education could modify its annual report on state and school district spending data to include obligations data in subsequent reporting cycles. Education agreed with this recommendation.
Small Business Assistance Programs
The Consolidated Appropriations Act, 2021, appropriated additional funding for the creation of the Targeted Economic Injury Disaster Loan (EIDL) Advance program and authorized additional Paycheck Protection Program (PPP) loans, among other things, highlighting the continued need for ensuring program integrity. Since March 2020, the Department of Justice has publicly announced charges in numerous fraud-related cases associated with loans made through these programs. As a result of concerns about program integrity, GAO has added Small Business Administration (SBA) loans to GAO’s High Risk List. SBA has taken some steps to mitigate fraud risks to EIDL and PPP, but it has not taken a strategic approach to managing fraud risks to both programs. GAO recommends that SBA (1) implement a comprehensive oversight plan to identify and respond to risk in the EIDL program to ensure program integrity, achieve program effectiveness, and address potential fraud; (2) conduct and document a fraud risk assessment for the EIDL program and PPP; (3) develop a strategy that outlines specific actions to address assessed fraud risks in the EIDL program; and (4) outline specific actions to monitor and manage fraud risks in PPP on a continuous basis. SBA agreed with these recommendations.
Unemployment Insurance Programs
GAO continues to have concerns about overpayments and potential fraud in the unemployment insurance (UI) system, including the federally funded Pandemic Unemployment Assistance (PUA) program, which authorizes UI benefits to certain individuals not otherwise eligible for these benefits, such as self-employed and certain gig economy workers. As of March 15, 2021, DOL reported that states had identified more than $3.6 billion in PUA overpayments from March 2020 through February 2021. In response to a recommendation in GAO’s January 2021 report, DOL has taken steps to collect data on states’ recovery of PUA overpayments. However, the Consolidated Appropriations Act, 2021, enacted in December 2020, provided states with authority to waive certain PUA overpayments. Thus, additional data on the amounts of PUA overpayments states have waived are also needed to effectively monitor the recovery of overpayments. GAO recommends that DOL collect data from states on the amount of overpayments waived in the PUA program, similar to the regular UI program. DOL agreed with this recommendation.
This report contains additional recommendations related to transparency and accountability in the following areas: relief for health care providers, economic impact payments, federal contracts and agreements, audits of nonfederal entities receiving federal pandemic assistance, and employer tax relief and payroll tax deferrals.
GAO is also examining the federal government’s COVID-19 vaccine efforts, which will be the focus of an upcoming report. Finally, GAO will review actions federal agencies have taken in response to the American Rescue Plan of 2021 in future reporting.
Why GAO Did This Study
As of March 15, 2021, the U.S. had over 29 million reported cases of COVID-19 and more than 523,000 reported deaths, according to CDC. The country also continues to experience serious economic repercussions.
Five relief laws, including the CARES Act, were enacted as of January 31, 2021, to provide appropriations to address the public health and economic threats posed by COVID-19. As of January 31, 2021, of the $3.1 trillion appropriated by these five laws for COVID-19 relief, the federal government had obligated a total of $2.2 trillion and expended $1.9 trillion, as reported by federal agencies.
Most recently, in March 2021, a sixth relief law, the American Rescue Plan of 2021, was enacted and provides additional federal assistance for the ongoing response and recovery.
The CARES Act includes a provision for GAO to report on its ongoing monitoring and oversight efforts related to the COVID-19 pandemic. This report examines the federal government’s continued efforts to respond to and recover from the COVID-19 pandemic.
GAO reviewed data, documents, and guidance from federal agencies about their activities and interviewed federal and state officials, experts, and other stakeholders, including health care professionals.
Recommendations
GAO is making 28 new recommendations for agencies that are detailed in this Highlights and in the report.
Recommendations for Executive Action
| Agency Affected | Recommendation | Status |
|---|---|---|
| Department of Health and Human Services | The Secretary of Health and Human Services should make the Department's different sources of publicly available COVID-19 data accessible from a centralized location on the internet. This could improve the federal government's communication with the public about the ongoing pandemic. See Health Care Indicators enclosure. (Recommendation 1) |
HHS neither agreed nor disagreed with our recommendation. In April 2023, HHS stated that it had streamlined the availability of publicly available COVID-19 data by consolidating the data from multiple online locations to two online locations-the CDC COVID Data/HHS Protect Public Data Hub and the HealthData.gov website. The HealthData.gov website also includes data on other health care topics. In its July 2023 update, HHS stated that it automatically replicates all data from the CDC COVID Data/HHS Protect Public Data Hub to the HealthData.gov website and HHS provided screen shots from the two websites showing that they contain links to each other. We consider HHS's actions to meet the intent of our recommendation and therefore we are closing this recommendation as implemented.
|
| Department of Health and Human Services | The Secretary of Health and Human Services should finalize and implement a post-payment review process to validate COVID-19 Uninsured Program claims and to help ensure timely identification of improper payments, including those resulting from potential fraudulent activity, and recovery of overpayments. See Relief for Health Care Providers enclosure. (Recommendation 2) |
HHS agreed with our recommendation to finalize and implement a post-payment review process. In July 2021, HHS stated it was developing the post-payment review audit strategy for the Uninsured Program, which includes detailed protocol and procedures for the assessments of the Uninsured Program to be executed by audit contractors. In March 2022, HHS provided us a copy of its COVID-19 Uninsured Program Assessment Strategy Manual. The manual provides detailed protocols and procedures for assessing the Uninsured Program claims to identify overpayments for recovery. As such, we believe this manual addresses our recommendation.
|
| Department of Health and Human Services | The Secretary of Health and Human Services should ensure that the Director of the Centers for Disease Control and Prevention collects data specific to the COVID-19 vaccination rates in nursing homes and makes these data publicly available to better ensure transparency and that the necessary information is available to improve ongoing and future vaccination efforts for nursing home residents and staff. See Nursing Homes enclosure. (Recommendation 3) |
HHS neither agreed nor disagreed with our recommendation. In March 2021, HHS said it was working towards better data transparency and noted that nursing homes have an opportunity to voluntarily report data through the National Healthcare Safety Network tracking system. On May 13, 2021, CMS issued an interim final rule establishing Long-Term Care Facility Vaccine Immunization Requirements for Residents and Staff, including for nursing homes. The rule requires facilities to report COVID-19 vaccination status of residents and staff to CDC. According to CDC, the new vaccination reporting requirement will not only assist in monitoring vaccine uptake among residents and staff, but will also aid in identifying facilities that may be in need of additional resources and assistance to respond to the COVID-19 pandemic. As of June 10, 2021, CMS has posted resident and staff vaccination rates for over 15,000 Medicare and Medicaid certified nursing homes on a public COVID-19 Nursing Home Data tracking website.
|
| Department of Health and Human Services | The Secretary of Health and Human Services should ensure that the Administrator of the Centers for Medicare & Medicaid Services, in consultation with the Centers for Disease Control and Prevention, requires nursing homes to offer COVID-19 vaccinations to residents and staff and design and implement associated quality measures. See Nursing Homes enclosure. (Recommendation 4) |
: HHS neither agreed nor disagreed with our recommendation. The Centers for Medicare and Medicaid Services (CMS) subsequently issued several regulatory requirements related to COVID-19 vaccinations in nursing homes, including: On May 13, 2021, CMS issued an interim final rule that establishes new requirements for nursing homes to develop and implement policies and procedures for educating residents, their representatives, and staff members about the COVID-19 vaccine and for offering these vaccines to each resident and staff member. Facilities are assessed for compliance with the new requirements, which became effective on May 21, 2021. On August 13, 2021, CMS issued a final rule that includes, among other things, a new quality measure for long-term care hospitals, including skilled nursing facilities, called the COVID-19 Vaccination Coverage among Healthcare Personnel measure. The measure requires facilities to submit data on COVID-19 staff vaccination beginning on October 1, 2021, and is being used as part of CMS's quality reporting program beginning in fiscal year 2023. On April 4, 2023, CMS issued a proposed rule and on July 31, 2023 CMS issued a final rule in which CMS adopted a new Skilled Nursing Facility Quality Reporting Program measure called the COVID-19 Vaccine: Percent of Patients/Residents Who Are Up to Date measure. The new quality measure, which will be incorporated into the program beginning with fiscal year 2026, reports the percentage of stays in which residents in a nursing home are up to date with recommended COVID-19 vaccinations in accordance with the Centers for Disease Control and Prevention's guidance. We believe CMS's adoption of these regulatory requirements related to COVID-19 nursing home vaccinations including the adoption of associated quality measures, address our recommendation.
|
| Office of the Under Secretary for Health | The Department of Veterans Affairs Under Secretary for Health should develop metrics to assess the number of vaccines administered by vaccine rollout phase to better assess progress and make any necessary adjustments as needed. See Veterans Health Care enclosure. (Recommendation 5) |
VA agreed with our recommendation and stated that its goal is to vaccinate all eligible veterans and employees who want to be vaccinated in 2021. In June 2021, VA provided us with evidence that it is tracking the number of vaccines administered by priority group. For example, VA has metrics on the number of vaccinated and unvaccinated veterans aged 75 and older.
|
| Office of the Under Secretary for Health | The Department of Veterans Affairs Under Secretary for Health should develop preliminary vaccination targets for when it will move from one vaccination phase to another; or within one phase, from one group of veterans to another. See Veterans Health Care enclosure. (Recommendation 6) |
VA concurred in principle with our recommendation. VA told us that it did not independently develop vaccination targets for moving from one phase to another. Rather, according to VA, it followed CDC guidance which called for a phased approach and flexibility to ensure efficient use of vaccines while supply was limited. According to VA, it is no longer using a phased approach and vaccine supply in the United States is no longer limited. Therefore, we are closing our recommendation as not implemented because VA is no longer using a phased approach to administer vaccines.
|
| Office of the Under Secretary for Health | The Department of Veterans Affairs Under Secretary for Health should collect data on the number of staff and veterans who do not show up for a vaccination appointment to better monitor for completion of the second dose of the vaccine. See Veterans Health Care enclosure. (Recommendation 7) |
VA agreed with our recommendation. In June 2021, VA provided us with evidence that is tracking the number of vaccines administered by priority group, including VHA health care staff, VA staff, and veterans. For example, it's Veteran Outreach Tool includes data on vaccination status, including if a veteran is interested in receiving or refused vaccination, received a vaccine from VA or an outside provider, and if a veteran is overdue for their second dose of Moderna's or Pfizer's vaccine. According to VA, providers use these data for individual veteran outreach and scheduling. VA's actions meet the intent of our recommendation, which was to use data to track vaccine administration and target outreach to improve completion of vaccine regimens.
|
| Department of Health and Human Services | The Secretary of Health and Human Services should ensure that the Food and Drug Administration and the Centers for Disease Control and Prevention work with the Assistant Secretary of Labor for Occupational Safety and Health to develop a process for sharing information to facilitate decision-making and guidance consistency related to devices with emergency use authorization. See Emergency Use Authorizations for Medical Devices enclosure. (Recommendation 8) |
HHS concurred with the recommendation, and in April 2023, OSHA, FDA, and CDC established a Memorandum of Understanding to share information related to medical devices for the purposes of facilitating coordination, decision-making, law enforcement activities, and guidance or regulation development. According to the Memorandum, the agencies' experiences with COVID-19 emergency use authorizations for certain medical devices, i.e., personal protective devices, demonstrated the need for timely and consistent information-sharing between the agencies both during and outside public health emergencies. As such, and in response to our recommendation, the agencies established the Memorandum to address this information-sharing and enable expanded collaborative activities between the agencies.
|
| Occupational Safety and Health Administration | The Assistant Secretary of Labor for Occupational Safety and Health should work with the Food and Drug Administration and the Centers for Disease Control and Prevention to develop a process for sharing information to facilitate decision-making and guidance consistency related to devices with emergency use authorization. See Emergency Use Authorizations for Medical Devices enclosure. (Recommendation 9) |
DOL concurred with the recommendation, and in April 2023, OSHA, FDA, and CDC established a Memorandum of Understanding to share information related to medical devices for the purposes of facilitating coordination, decision-making, law enforcement activities, and guidance or regulation development. According to the Memorandum, the agencies' experiences with COVID-19 emergency use authorizations for certain medical devices, i.e., personal protective devices, demonstrated the need for timely and consistent information-sharing between the agencies both during and outside public health emergencies. As such, and in response to our recommendation, the agencies established the Memorandum to address this information-sharing and enable expanded collaborative activities between the agencies.
|
| Food and Drug Administration | As the Food and Drug Administration develops a transition plan for devices with emergency use authorizations, the Commissioner should specify a reasonable timeline and process for transitioning authorized devices to clearance, approval, or appropriate disposition that takes into account input from stakeholders. See Emergency Use Authorizations for Medical Devices enclosure. (Recommendation 10) |
HHS concurred with this recommendation. In March 2021, FDA stated that it believes it is important to provide such a transition period to allow sponsors to meet any additional requirements. In addition, FDA stated it will provide the transition plan in the form of draft guidance for public comment so the agency can work to incorporate suggestions from those impacted by the transition. In May 2021, FDA reiterated its intent to address our recommendation and the agency's plan to issue draft guidance. FDA stated that given volume of device EUAs, the agency recognizes the need for transparency regarding the timeline and approach for transitioning from EUA to marketing authorization. In December 2021, FDA issued a "Transition Plan for Medical Devices Issued Emergency Use Authorizations During the Coronavirus Disease 2019 Public Health Emergency: Draft Guidance for Industry and FDA Staff". The guidance outlines both a timeline for advance notice of termination of EUA declarations and recommendations for manufacturers to prepare marketing submission for clearance or approval of devices when they wish to continue distributing devices with EUAs. FDA is seeking comment from stakeholders on the draft guidance through March 23, 2022. FDA's actions fulfill the intent of our recommendation.
|
| Centers for Disease Control and Prevention | The Director of the Centers for Disease Control and Prevention should incorporate key elements of a national strategy in the agency's COVID-19 Response Health Equity Strategy. These elements include (1) specific actions to achieve intermediate outcomes, such as increased access to testing; (2) how intermediate outcomes should be prioritized within its four broad priority areas; (3) who will implement actions to achieve intermediate outcomes; and (4) how the strategy relates to other relevant strategies. See Health Disparities enclosure. (Recommendation 11) |
CDC agreed with this recommendation. In May 2021, CDC reported that it had implemented the Health Equity Action Tracker as an internal repository of health equity activities related to COVID-19. According to CDC, this tracker includes questions about alignment with the intermediate outcomes in the CDC Health Equity Strategy, as well as questions about additional outcomes and the impact of the activities. In August 2021, CDC stated that it added 108 projects and activities to its tracker from multiple task forces including state, tribal, local, and territorial and community interventions and critical populations task forces. In addition, the agency reported that it launched a Health Equity in Action website in April 2021 to share activities, partnerships, and resources to advance health equity and continues to add resources to this website. For example, CDC added information on working with trusted organizations to engage non-Hispanic Black communities at higher risk for COVID-19 illness and death to adopt and sustain COVID-19 preventive and community mitigation strategies. Additionally, in January 2022, CDC provided examples of health equity activities task forces undertook intended to achieve intermediate strategy outcomes. For example, in Summer 2021, CDC participated in radio interviews in Spanish and English to answer questions on COVID-19 vaccines in alignment with the Health Equity Strategy priority to increase the number of effective culturally and linguistically tailored programs for COVID-19. Furthermore, in January 2022, CDC described connections between the Health Equity Strategy and other relevant strategies such as the National Strategy for the COVID-19 Response and Pandemic Preparedness. For example, both strategies include goals to protect vulnerable populations and advance equity in COVID-19 response efforts.
|
| Centers for Disease Control and Prevention | The Director of the Centers for Disease Control and Prevention should take steps to ensure more complete reporting of race and ethnicity information for recipients of COVID-19 vaccinations, such as working with states and jurisdictions to facilitate consistent collecting and reporting of this information. See Health Disparities enclosure. (Recommendation 12) |
CDC neither agreed nor disagreed with this recommendation. In March 2021, CDC stated that it was working to ensure more complete reporting of race and ethnicity information for recipients of COVID-19 vaccinations, such as by requiring providers that participate in CDC's COVID-19 Vaccination Program to report the race and ethnicity of vaccine recipients. In August 2021, CDC stated the agency continues to work with states and jurisdictions to improve demographic data completeness and noted that it is seeing improvements with electronic reporting through CDC's Data Modernization Initiative. CDC stated they send weekly data quality reports to every jurisdiction, federal entity, and pharmacy to share progress in achieving key data reporting elements like race and ethnicity. CDC stated that it provides technical assistance to raise awareness about race and ethnicity data quality issues to ensure systems are able to collect the required data elements and to help address jurisdictions' and partners' concerns about privacy issues. CDC also stated that it began awarding funding to 107 health departments specifically focusing on reducing health disparities related to COVID-19, including to improve data collection and reporting of racial and ethnic information. In January 2022, CDC stated the agency began sending weekly data quality reports to jurisdictions in February 2021 and provides tips and targets for improving data collection, including for race and ethnicity. CDC stated that jurisdictions improved data collection and the agency reported decreases in incomplete race and ethnicity data for recipients of COVID-19 vaccines. For example, in April 2022, CDC reported that the percentage of COVID-19 vaccinations with known ethnicity had increased from 79 percent to 87 percent between February and December of 2021.
|
| Department of Agriculture | The Secretary of Agriculture should direct the Administrator of the Agricultural Marketing Service to issue guidance—such as an acquisition alert or a reminder to contracting officials—on the use of the COVID-19 National Interest Action code for the Farmers to Families Food Box Program or successor food distribution program to ensure it accurately captures COVID-19-related contract obligations in support of the program. See Federal Contracts and Agreements for COVID-19 enclosure. (Recommendation 13) |
The U.S. Department of Agriculture neither agreed nor disagreed with our recommendation. In February 2021, following our identification of contract data reporting challenges using the COVID-19 National Interest Action code for the Farmers to Families Food Box Program, Agricultural Marketing Service officials said they conducted training with staff to review National Interest Action code data entry protocols. At that time, a senior Agricultural Marketing Service official also sent an email reminder to procurement division personnel about OMB's guidance on the use of the COVID-19 National Interest Action code. Following this training and email, officials took action to retroactively report contract actions for the program with the National Interest Action code. In May 2021, the Agricultural Marketing Service updated its instructions for entering contract actions into the Federal Procurement Data System-Next Generation to include a reminder to utilize the proper National Interest Action code, if applicable.
|
| Department of Agriculture | The Secretary of Agriculture should direct the Administrator of the Agricultural Marketing Service to assess the contracting personnel needed to fully execute the award and administration of existing contracts in support of the Farmers to Families Food Box Program or successor future food distribution program, and take the necessary steps to ensure it has adequate contracting staff in place to award and administer any future contracts for the program. See Federal Contracts and Agreements for COVID-19 enclosure. (Recommendation 14) |
USDA neither agreed nor disagreed with our recommendation, and according to Agricultural Marketing Service officials, they have discontinued the program, and are using other methods of hunger relief, so they do not anticipate needing additional permanent staff. However, in December 2021, the Agricultural Marketing Service placed an order on an existing contract vehicle to obtain additional staff support for contract closeout services and other administrative needs for the awards that have been made under the Farmers to Families Food Box Program and other food purchasing efforts. The statement of work for the order estimates the number of contracts the Agricultural Marketing Service needs contract support services for, and required the contractor to deliver a staffing plan, identifying how it will meet USDA's requirements. The contractor's April 2022 staffing plan identifies a total of three additional staff to lead and support Agricultural and Marketing Services contract closeout needs for the Farmers to Families Food Box Program. The plan also states that staffing needs will be reassessed and additional resources acquired as needed, which will help to ensure that Agricultural and Marketing Services has the needed staff support to finalize the closeout of the contracts.
|
| Department of Labor | The Secretary of Labor should ensure the Office of Unemployment Insurance collects data from states on the amount of overpayments waived in the Pandemic Unemployment Assistance program, similar to the regular unemployment insurance program. See Unemployment Insurance Programs enclosure. (Recommendation 15) |
DOL agreed with our recommendation and on September 3, 2021, issued PUA program guidance and updated instructions for states to report PUA overpayments waived. As of June 2023, all 53 states and territories have reported some data on the amount of PUA overpayments waived in any month, though some have reported zero amounts waived. DOL will continue to monitor all states' and territories' data to ensure that their reporting of PUA overpayments waived is accurate.
|
| Internal Revenue Service | The Commissioner of Internal Revenue should periodically review control activities for issuing direct payments to individuals to determine that the activities are designed and implemented appropriately as IRS disburses a third round of Economic Impact Payments and prepares for advance payments on the Child Tax Credit. These control activities should include appropriate testing procedures, quality assurance reviews, and processes that ensure payments distributed by tax partners reach the intended recipients. See Economic Impact Payments enclosure. (Recommendation 16) |
IRS took steps to implement our recommendations, such as updating control procedures for issuing direct payments to individuals. Additionally, individuals had the opportunity to update their bank account information during the 2021 filing season, which ran from February 12 through May 17, 2021. IRS officials said that the updated procedures resulted in a low number of EIP 3 payments being sent to incorrect bank accounts. Additionally, officials anticipated the same for July 2021 advance CTC payments. The number of direct payments that were rejected for EIP 2 was over 5.3 million and for EIP 3 it was over 2.5 million. Additionally, for the July 2021 advance CTC payments, over 500,000 direct payments were rejected.
|
| Department of Agriculture | The Secretary of Agriculture should ensure that the Administrator of the Food and Nutrition Service (1) provides sufficient context to help stakeholders and the public understand and interpret data on federal nutrition assistance programs during the pandemic and (2) discloses potential sources of error that may affect data quality during the pandemic, such as manual processing. For example, the agency could publish key information from its internal communications plan that it developed for the January 2021 data release and include additional table notes in subsequent data releases to help explain these issues. See Nutrition Assistance enclosure. (Recommendation 17) |
FNS generally agreed with this recommendation and took action to address it. In March 2021, FNS reconfigured its data system so that manual processing would no longer be necessary. By doing so, FNS has removed potential sources of error that affected data quality earlier in the COVID-19 pandemic. The agency also added several table notes to data it released in April 2021 to help provide stakeholders and the public with sufficient context to understand and interpret key data.
|
| Internal Revenue Service | The Commissioner of Internal Revenue should leverage employee counts from Form 941, Employer's Quarterly Federal Tax Return, and Form 943, Employer's Annual Federal Tax Return for Agricultural Employees, to identify potentially ineligible COVID-19 related sick and family leave credit claims, and address discrepancies the Internal Revenue Service deems significant. See Employer Tax Relief and Payroll Tax Deferrals enclosure. (Recommendation 18) |
IRS implemented our recommendation. Specifically, IRS's September 2021 compliance plan states that the agency will use Form 941 and Form 943 line 1 data in conjunction with W-2, Wage and Tax Statement, information and additional data to identify potentially ineligible COVID-19 related credit claims for possible examination. Leveraging available tax data will improve the effectiveness of IRS's efforts to ensure ineligible claimants do not keep leave credit benefits they were not entitled to.
|
| Internal Revenue Service | The Commissioner of Internal Revenue should conduct outreach to employment tax return filers to educate and promote accurate reporting of employee counts on Form 941, Employer's Quarterly Federal Tax Return, and Form 943, Employer's Annual Federal Tax Return for Agricultural Employees. See See Employer Tax Relief and Payroll Tax Deferrals enclosure. (Recommendation 19) |
In May 2021, IRS released a "tax tip" for employment tax return filers reminding them to ensure that line 1 of their return is accurate and referring employers to the form instructions for details. This information could support compliance efforts, which can result in multiple benefits, including helping taxpayers understand their responsibilities for tax compliance and decreasing potentially ineligible credit claims.
|
| Small Business Administration |
Priority Rec.
The Administrator of the Small Business Administration should conduct and document a fraud risk assessment for the Economic Injury Disaster Loan program. See Economic Injury Disaster Loan Program enclosure. (Recommendation 20) |
SBA agreed with the recommendation. In December 2021, SBA provided a fraud risk assessment for the program that had been prepared by its contractor. This assessment adhered to many fraud risk management leading practices, but SBA did not determine its fraud risk tolerance as called for by leading practices. In February 2022, SBA designated an antifraud entity that, according to SBA officials, would be responsible for determining a risk tolerance and implementing the fraud risk assessment's recommendations. In April 2022, SBA created a fraud risk tolerance for EIDL that identified potential risks that exceeded SBA's willingness to tolerate them. SBA also identified mitigating activities to minimize those risks that exceeded SBA's tolerance.
|
| Small Business Administration |
Priority Rec.
The Administrator of the Small Business Administration should develop a strategy that outlines specific actions to address assessed fraud risks in the Economic Injury Disaster Loan program on a continuous basis. See Economic Injury Disaster Loan Program enclosure. (Recommendation 21) |
SBA agreed with the recommendation. In August 2023, SBA finalized a fraud risk strategy for the program that identified approaches to prevent, detect, and respond to instances of active and potential fraud in COVID EIDL. For example, the document describes SBA's efforts to use advanced data analytics, machine learning technologies, and cross-program data analytics to screen for potential fraud and ineligibility.
|
| Small Business Administration |
Priority Rec.
The Administrator of the Small Business Administration should implement a comprehensive oversight plan to identify and respond to risks in the Economic Injury Disaster Loan program to help ensure program integrity, achieve program effectiveness, and address potential fraud. See Economic Injury Disaster Loan Program enclosure. (Recommendation 22) |
In August 2022, SBA provided an updated oversight plan it developed for the EIDL program. The plan identified controls SBA had or planned to implement for COVID-EIDL loans. For example, SBA noted that it began conducting fraud and identity theft reviews on a small scale of loans at the start of the COVID-EIDL program, expanded the reviews in June 2020, and continued to conduct such reviews on a sample of disbursed loans. In November 2022, SBA noted that it was focusing its resources on the COVID-EIDL servicing phase because loans were no longer being approved under the COVID-EIDL program and there were very limited funds left to disburse. During this phase, SBA noted that it would only conduct loan reviews on non-performing loans and servicing requests. In February 2023, SBA provided additional documentation regarding these reviews. For example, SBA will conduct manual reviews on loans that become 90-day delinquent to confirm whether any agency holds exist for suspected fraud or identity theft. In addition, SBA will conduct such reviews for servicing requests (e.g., collateral adjustments, loan modifications, and hardship reviews) before approving them. SBA also noted that it would offer COVID-EIDL borrowers hardship reduction payments (10 percent of monthly payment) to try to encourage regular payments and maximize the recovery of taxpayer funds.
|
| Small Business Administration |
Priority Rec.
The Administrator of the Small Business Administration should conduct and document a fraud risk assessment for the Paycheck Protection Program. See Paycheck Protection Program enclosure. (Recommendation 23) |
SBA agreed with the recommendation. In December 2021, SBA provided a fraud risk assessment for the program that had been prepared by its contractor. This assessment adhered to many fraud risk management leading practices, but SBA did not determine its fraud risk tolerance as called for by leading practices. In February 2022, SBA designated an antifraud entity that, according to SBA officials, would be responsible for determining a risk tolerance and implementing the fraud risk assessment's recommendations. In April 2022, SBA created a fraud risk tolerance for PPP that identified potential risks that exceeded SBA's willingness to tolerate them. SBA also identified mitigating activities to minimize those risks that exceeded SBA's tolerance.
|
| Small Business Administration |
Priority Rec.
The Administrator of the Small Business Administration should develop a strategy that outlines specific actions to monitor and manage fraud risks in the Paycheck Protection Program on a continuous basis. See Paycheck Protection Program enclosure. (Recommendation 24) |
SBA agreed with the recommendation. In August 2023, SBA finalized a fraud risk strategy for the program that identified approaches to prevent, detect, and respond to instances of active and potential fraud in the PPP. For example, the document describes SBA's efforts to use data analytics methods to review the PPP loan portfolio with the intent of managing fraud risks by reducing false positives, prioritizing identified fraud typologies and behaviors, and uncovering areas of fraud risk not previously identified. .
|
| Federal Emergency Management Agency | The Federal Emergency Management Agency Administrator should adhere to the agency's protocols listed in its updated 2019 Tribal Consultation Policy by obtaining tribal input via the four phases of the tribal consultation process when developing new policies and procedures related to COVID-19 assistance. See FEMA's Disaster Relief Fund and Assistance to Tribal Governments enclosure. (Recommendation 25) |
In March 2021, DHS concurred with our recommendation. DHS states that FEMA's National Tribal Affairs Advisor, based in the Office of External Affairs, will coordinate with other FEMA offices and directorates, as appropriate, to review the agency's adherence to protocols listed in the Tribal Consultation policy. According to FEMA officials, in March 2021, FEMA conducted formal consultation with Tribal Leaders on COVID-19 Funeral Assistance before finalizing the interim policy. In April 2021, FEMA sent letters to tribal leaders discussing (1) FEMA policy and procedure for financial assistance to individuals and households for COVID-19 related funeral expenses incurred after January 20, 2020; and (2) a framework, policy details and requirements for determining the eligibility of safe opening and operation work and costs under the Public Assistance program. According to FEMA officials, as of March 2022, no additional COVID-related policies or regulations are under development. As a result, we consider this recommendation as closed-implemented.
|
| Federal Emergency Management Agency | The Federal Emergency Management Agency Administrator should provide timely and consistent technical assistance to support tribal governments' efforts to request and receive Public Assistance as direct recipients, including providing additional personnel, if necessary, to ensure that tribal nations are able to effectively respond to COVID-19. See FEMA's Disaster Relief Fund and Assistance to Tribal Governments enclosure. (Recommendation 26) |
In March 2021, DHS concurred with our recommendation. DHS stated that FEMA's Recovery Directorate would publish a memorandum that will contain direction to FEMA regions regarding the assignment of Public Assistance Program delivery managers to promote equitable delivery of Public Assistance to tribal governments. According to FEMA officials, in August 2021, FEMA sent a memorandum that provided updates on how FEMA would deliver assistance. This guidance provides FEMA's regional staff the ability to work with all tribal applicants to understand their capacity to address issues through their assigned Public Assistance program delivery manager. FEMA also stated that in September 2021, it hosted a webinar to further enhance staff readiness to deliver direct technical assistance and support to tribes. Further FEMA stated that as of December 2021, 223 out of 355 of the tribal entities that are eligible Public Assistance applicants have requested and received a program delivery manager. During the winter of 2022, we reached out to representatives from the tribal governments to see if the assistance provided by these program delivery managers has been reliable and generally has addressed their needs and concerns. According to two organizations we met with, communication with FEMA has been unreliable, so they could not say that FEMA has improved its technical assistance in a timely or consistent manner. FEMA continues to take actions to address our recommendation. In August 2022, FEMA issued its National Tribal Strategy that outlines its major strategic goals and objectives in working with tribal nations. It includes information requested and recommended by tribal nations through outreach sessions. FEMA added issue areas to address requests from Tribal Nation members, to include tribal-specific technical assistance and tailored resources to support tribal emergency management programs. In October 2022, FEMA provided information by region on their outreach efforts and examples of assistance provided to tribal nations. As a result of these actions we consider this recommendation closed and implemented.
|
| Office of the Secretary of the Department of Education |
Priority Rec.
The Secretary of Education should regularly collect and publicly report information on school districts' financial commitments (obligations), as well as outlays (expenditures) in order to more completely reflect the status of their use of federal COVID-19 relief funds. For example, Education could modify its annual report on state and school district spending data to include obligations data in subsequent reporting cycles. See K-12 Education enclosure. (Recommendation 27) |
The Department of Education (Education) agreed with GAO's recommendation and has taken a number of steps to work with states to provide greater clarity on state and school district spending. As of January 4th, Education updated its data collection instrument on the status of COVID-19 relief funding to include budgeted or earmarked uses of remaining funds (obligations) for each of the three Education Stabilization Relief funds. Beginning in Spring 2022, these data will be collected annually until all funds have been liquidated. Following a data quality review period, Education will publish descriptive statistics based on this information on the Education Stabilization Fund (ESF) Public Transparency Portal no later than spring/summer of 2022, and on a similar time frame in subsequent years.
|
| Office of Management and Budget |
Priority Rec.
The Director of the Office of Management and Budget should work in consultation with federal agencies and the audit community (e.g., agency Offices of Inspector General; National Association of State Auditors, Comptrollers, and Treasurers; and American Institute of Certified Public Accountants), to the extent practicable, to incorporate appropriate measures in the Office of Management and Budget's process for preparing single audit guidance, including the annual Single Audit Compliance Supplement, to better ensure that such guidance is issued in a timely manner and is responsive to users' input and needs. See Single Audits enclosure. (Recommendation 28) |
OMB issued its Compliance Supplement in May of 2022, 2023, and 2024. During the development of the 2024 Supplement, OMB provided drafts of the Supplement and held meetings with the single audit community to obtain feedback. OMB also worked with the federal agencies by holding a 2024 Supplement kick-off meeting and providing a Preparation Guide, which includes guidance and a schedule for providing input on the 2024 Supplement. OMB's actions to provide more timely single audit guidance and collaborate with the audit community early in the process addressed our recommendation. Timely guidance can help ensure that single audits can be performed, which helps enhance the federal government's ability to safeguard billions of dollars in federal funds.
|
