COVID-19: Federal Efforts Accelerate Vaccine and Therapeutic Development, but More Transparency Needed on Emergency Use Authorizations
Fast Facts
The federal government, in concert with private industry, has greatly accelerated efforts to develop vaccines and therapeutics for COVID-19 through Operation Warp Speed. This partnership is spending more than $10 billion on 6 vaccine candidates.
In a separate effort to speed access to medical products, the Food and Drug Administration has so far issued four emergency use authorizations, referred to as EUAs, that temporarily allow use of unapproved therapeutics. Its rationale for doing so has not always been clear. To help ensure public trust, we recommended the FDA better communicate the findings of its safety and effectiveness reviews.
Highlights
What GAO Found
Through Operation Warp Speed—a partnership between the Department of Health and Human Services (HHS) and the Department of Defense (DOD)—the federal government is accelerating efforts to develop vaccines and therapeutics for COVID-19. A typical vaccine development process can take approximately 10 years or longer, but efforts under Operation Warp Speed seek to greatly accelerate this process by completing key steps simultaneously (see figure). As of October 15, 2020, Operation Warp Speed publicly announced financial support for the development or manufacturing of six COVID-19 vaccine candidates totaling more than $10 billion in obligations. It has also announced financial support for the development of therapeutics, such as a $450 million award to manufacture a monoclonal antibody treatment (a treatment that uses laboratory-made antibodies, which also may be able to serve as a prevention option).
Operation Warp Speed Timeline for a Potential Vaccine Candidate
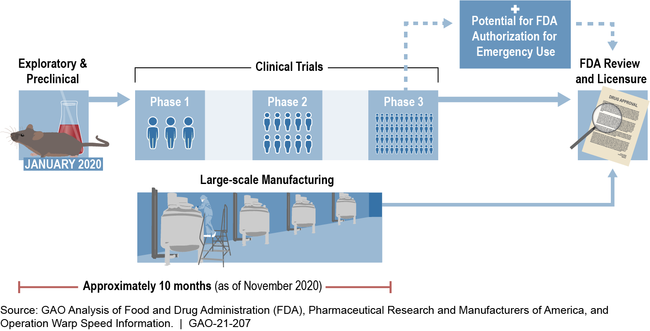
Note: An Emergency Use Authorization allows for emergency use of medical products without FDA approval or licensure during a declared emergency, provided certain statutory criteria are met.
The Food and Drug Administration (FDA) may temporarily allow the use of unlicensed or unapproved COVID-19 vaccines and therapeutics through emergency use authorizations (EUA), provided there is evidence that the products may be effective and that known and potential benefits outweigh known and potential risks. For vaccines, FDA issued guidance in October 2020 to provide vaccine sponsors with recommendations regarding the evidence FDA needed to support issuance of an EUA. For therapeutics, FDA has issued four EUAs as of November 9, 2020. The evidence to support FDA's COVID-19 therapeutic authorization decisions has not always been transparent, in part because FDA does not uniformly disclose its scientific review of safety and effectiveness data for EUAs, as it does for approvals for new drugs and biologics. Given the gravity of the pandemic, it is important that FDA identify ways to uniformly disclose this information to the public. By doing so, FDA could help improve the transparency of, and ensure public trust in, its EUA decisions.
Why GAO Did This Study
The U.S. had about 10.3 million cumulative reported cases of COVID-19 and about 224,000 reported deaths as of November 12, 2020. Given this catastrophic loss of life as well as the pandemic's effects on the U.S. economy, effective and safe vaccines and therapeutics are more important than ever.
The CARES Act includes a provision for GAO to report on its ongoing monitoring and oversight efforts related to the COVID-19 pandemic. This report examines, (1) efforts of Operation Warp Speed to accelerate COVID-19 vaccine and therapeutic development; and (2) FDA's use of EUAs for COVID-19 therapeutics and vaccines, among other objectives.
GAO reviewed federal laws and agency documents, including HHS and DOD information on vaccine and therapeutic development and EUAs as of November 2020. GAO interviewed or received written responses from HHS and DOD officials, and interviewed representatives from vaccine developers and manufacturers, as well as select public health stakeholders and provider groups covering a range of provider types.
Recommendations
FDA should identify ways to uniformly disclose to the public the information from its scientific review of safety and effectiveness data when issuing EUAs for therapeutics and vaccines. HHS neither agreed nor disagreed with the recommendation, but said it shared GAO's goal of transparency and would explore approaches to achieve this goal.
Recommendations for Executive Action
| Agency Affected | Recommendation | Status |
|---|---|---|
| Food and Drug Administration | The Secretary of Health and Human Services should direct the FDA Commissioner to identify ways to uniformly disclose to the public the information from FDA's scientific review of safety and effectiveness data—similar to the public disclosure of the summary safety and effectiveness data supporting the approval of new drugs and biologics—when issuing EUAs for therapeutics and vaccines, and, if necessary, seek the authority to publicly disclose such information. (Recommendation 1) |
In response to our recommendation, FDA said it would explore approaches to achieve the goal of transparency. On November 17, 2020, FDA made an announcement on the agency's ongoing commitment to transparency for COVID-19 EUA. FDA also developed a process to disclose its scientific review documents for therapeutic EUAs and released such summaries for one previous therapeutic EUA and the two additional therapeutic EUAs issued since our recommendation. These summaries disclosed information similar to what FDA releases to support new drug approvals and biologic licensures. Additionally, for the two vaccine EUAs FDA issued since our recommendation, FDA released decision memos containing detailed information about FDA's review of safety and effectiveness data. FDA's actions meet the intent of our recommendation and will improve transparency.
|
