Science & Tech Spotlight: COVID-19 Testing
Fast Facts
This Spotlight looks at the 3 types of FDA-authorized COVID-19 tests.
Molecular and antigen tests help diagnose active infections. Molecular tests look for the virus’s genetic material, while antigen tests look for unique parts of the virus. Results can be used for quarantines, routing supplies, etc.
Serology tests look for antibodies in the blood to help determine prior exposure.
So far, FDA has authorized at least 56 molecular tests and 12 serology tests for emergency use. It recently authorized one antigen test as well. Accuracy varies among all of the tests. The resulting uncertainty can complicate public health decisions.
A COVID-19 molecular test kit from CDC

Test kit
Highlights
Why This Matters
As the nation works to save lives and flatten the curve of COVID-19 cases, widespread testing could help reduce the death rate, help identify those who should self-isolate, and help businesses recover. There are challenges, however, associated with the use and availability of tests, as well as with determining their accuracy.
The Technology
What is it? There are currently three types of tests for COVID-19: molecular, antigen, and serology. Molecular tests and a new antigen test help diagnose active COVID-19 infections, and serology tests help determine whether someone was previously exposed to the novel coronavirus, which causes COVID-19.
How does it work? While each kind of test takes a different approach, they all collect vital information about exposure to the virus.
Molecular tests amplify and detect genetic material from novel coronavirus in a sample taken from a patient’s saliva, nose, or throat to determine whether there is an active infection (fig. 1). For example, molecular tests based on the polymerase chain reaction (PCR) use a genetic photocopier, copying a unique portion of the coronavirus genetic material, if present, until there are enough copies to detect.
Antigen tests can detect the virus by binding parts of the virus, called antigens, to a membrane. Antibodies that can fluoresce are also added to the membrane. If enough antigen is present to bind to the antibodies, the total fluorescence from the antibodies becomes high enough to indicate a positive result.
Serology tests, which typically require a sample of blood from a patient, can detect the presence of antibodies produced by a patient’s immune system in response to the coronavirus (fig. 1). Such antibodies can persist in the blood after a patient recovers from an infection. Thus, a positive serology test result indicates prior exposure to the novel coronavirus but does not necessarily mean that the patient is still infected.
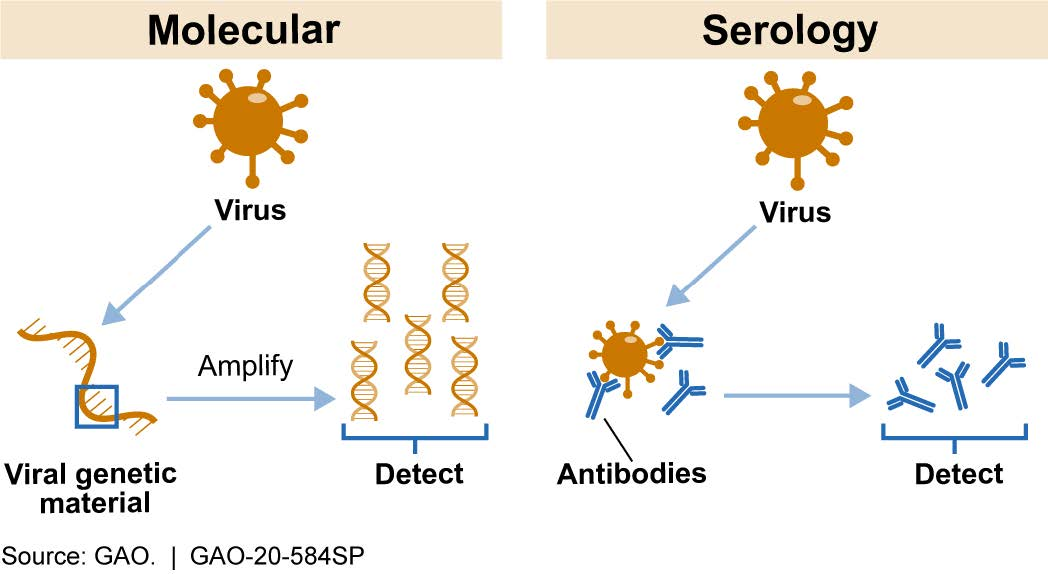
Figure 1. Most Common COVID-19 Test Types. Molecular tests detect viral genetic material. Serology tests detect antibodies against the virus.
How mature is it? All three kinds of COVID-19 tests are commercially available under the Food and Drug Administration’s (FDA’s) emergency use authorization (EUA), which allows for the use of certain unapproved medical products or unapproved uses of approved medical products under certain circumstances during a public health emergency. As of May 13, 2020, FDA has authorized at least 56 molecular tests for COVID-19 via EUA; these tests generally rely on PCR technology. Devices used for administering molecular tests range in size from larger equipment to smaller, portable devices that can be used on-site near the patient.
On May 8, 2020, FDA authorized the first antigen test for COVID-19 via EUA. According to FDA, antigen tests, which can be run in minutes, have a higher chance of false negatives compared to molecular tests. FDA states that negative antigen test results may need to be confirmed with a molecular test before making treatment decisions.
As of May 13, 2020, FDA has authorized at least 12 serology tests for COVID-19 via EUA. For COVID-19, serology tests were first authorized by FDA about a month after molecular tests were authorized. Devices used to administer serology tests also range in size, with some about the size of an over-the-counter pregnancy test.
Opportunities
Testing provides critical information for a variety of purposes, including medical care, policymaking, and business. For example:
- Determining risk. Serology tests can help establish the percentage of the population previously exposed to the virus. Such information can help improve the accuracy of statistics on infection rates, among other things
- Understanding disease impact. Testing can help establish the impact of the disease, including identifying those who may have been exposed to the virus but did not show symptoms and those who recovered. This is important for characterizing the risk of the disease to the elderly and other subpopulations.
- Guiding treatment and resource allocation. Molecular and antigen tests can provide information on the rate and geographic location of active infections. This can help guide patient treatment and inform decisions about the distribution of medical supplies and equipment.
- Informing responses. Testing is also important for informing decisions on quarantine and isolation, including decisions related to health care workers. Additionally, testing can help inform public responses such as school closures and reopenings.
Challenges
Although widespread testing offers a number of opportunities, there are also challenges in implementation. For example:
- Limited information on test accuracy. The performance characteristics of some COVID-19 tests have raised concerns about test accuracy. In particular, available data suggest variability in the rates of false positive and false negative results. This uncertainty can complicate patient treatment and public health decisions.
- Number and timing of test results. Centralized laboratories generally have higher reported testing throughput, but may take days to report test results. Point-of-care testing conducted on-site can return results more quickly, sometimes within an hour. However, the throughput of these tests can be lower than that of central laboratories. It may be challenging to balance the need for fast results and the need for large numbers of tests (fig. 2).
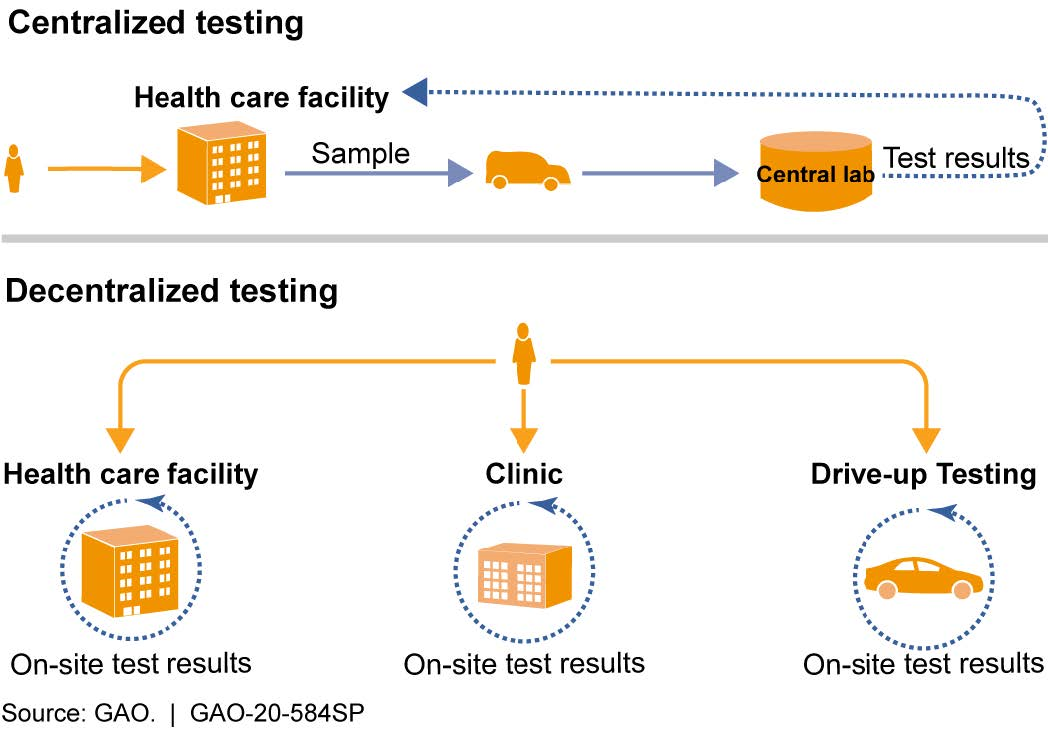
Figure 2. Types of test processing based on site location. Decentralized testing, including testing at the point-of-care, reduces the time to obtain test results but can have limited throughput and other challenges. Centralized testing can have higher throughput, but may take longer to provide test results.
- Scalability. Expanding testing to reach more people would require greater availability of resources such as personal protective equipment needed by healthcare workers. Another option for expanding testing may be for FDA to authorize the use of additional at-home sample collection kits. Allowing consumers totake their own samples, however, could result in reduced test accuracy, due to sample contamination or other issues.
Policy Context and Questions
There are a number of important policy issues related to COVID-19 testing. For example:
- What are the barriers, if any, to FDA’s and CDC’s ability to help facilitate the development and use of tests during an early stage of an outbreak, and how could any barriers be addressed?
- What actions could help the general public and health care workers understand and assess the accuracy and reliability of different tests?
- What information could help decision-makers determine the number and distribution of tests to most effectively respond to outbreaks such as the COVID-19 pandemic?
- How can federal, state, and local health departments coordinate to facilitate accurate and timely testing and reporting of test results?
For more information, contact Karen Howard at (202) 512-6888 or HowardK@gao.gov.
