Science & Tech Spotlight: CRISPR Gene Editing
Fast Facts
CRISPR is a gene editing technology that allows scientists to make specific, targeted changes to DNA.
Scientists are using CRISPR to develop treatments for medical conditions, including blindness and some cancers, as well as to create tests to rapidly identify COVID-19 and other infections. CRISPR may also be used to strengthen crops, develop new biofuels, and more.
As with any gene editing technology, unintended consequences are possible. For example, if CRISPR targets an unintended location within the DNA, edits could lead to disease. Many countries are struggling with how to regulate these technologies.
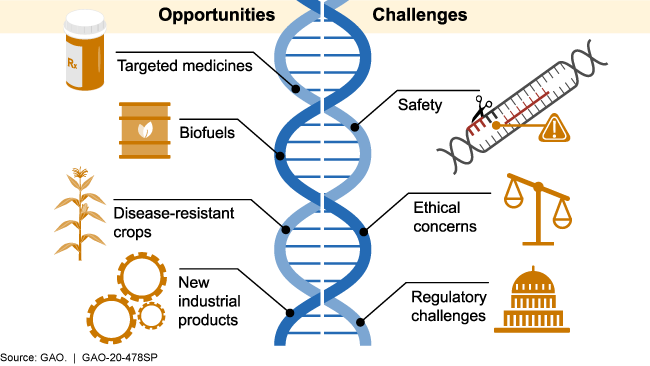
Illustration showing opportunities and challenges
Highlights
Why This Matters
CRISPR allows scientists to change DNA more quickly and efficiently than previous methods. Thousands of laboratories use it to research the potential development of new crops, medicines, and other advances that could have a significant impact. As a result, it has received widespread attention. However, there are safety, ethical, and regulatory concerns.
The Technology
What is it?
CRISPR is one type of gene editing technology that allows scientists to modify DNA, the hereditary information in humans and almost all other organisms. Modifying DNA can lead to changes in an organism’s characteristics, such as eye color or susceptibility to disease.
How does it work?
CRISPR leverages a natural defense mechanism of bacteria to cut DNA at a specific location. Specifically, when bacteria are attacked by a virus, they record a section of the virus’s DNA in their own DNA, bookending it with a repeating sequence called Clustered Regularly Interspaced Palindromic Repeats (CRISPR). Storing part of the virus’s genetic code allows the bacteria to “remember” it. When the same type of virus attacks again, the bacteria use a specific CRISPR-associated protein number 9 (CAS9) to cut the virus’s DNA, destroying the virus.
In the laboratory, scientists use this same CRISPR/CAS9 system to identify and cut a specific DNA sequence. They do this by creating an RNA sequence that matches the DNA they want to edit. (RNA may also carry genetic information.) Then, using this RNA sequence to guide the CAS9 protein to that location, scientists can effectively reprogram CAS9 to cut DNA at any desired location. Once CRISPR/CAS9 cuts the specific DNA that has been targeted, scientists use other techniques to add, delete, or modify the DNA.
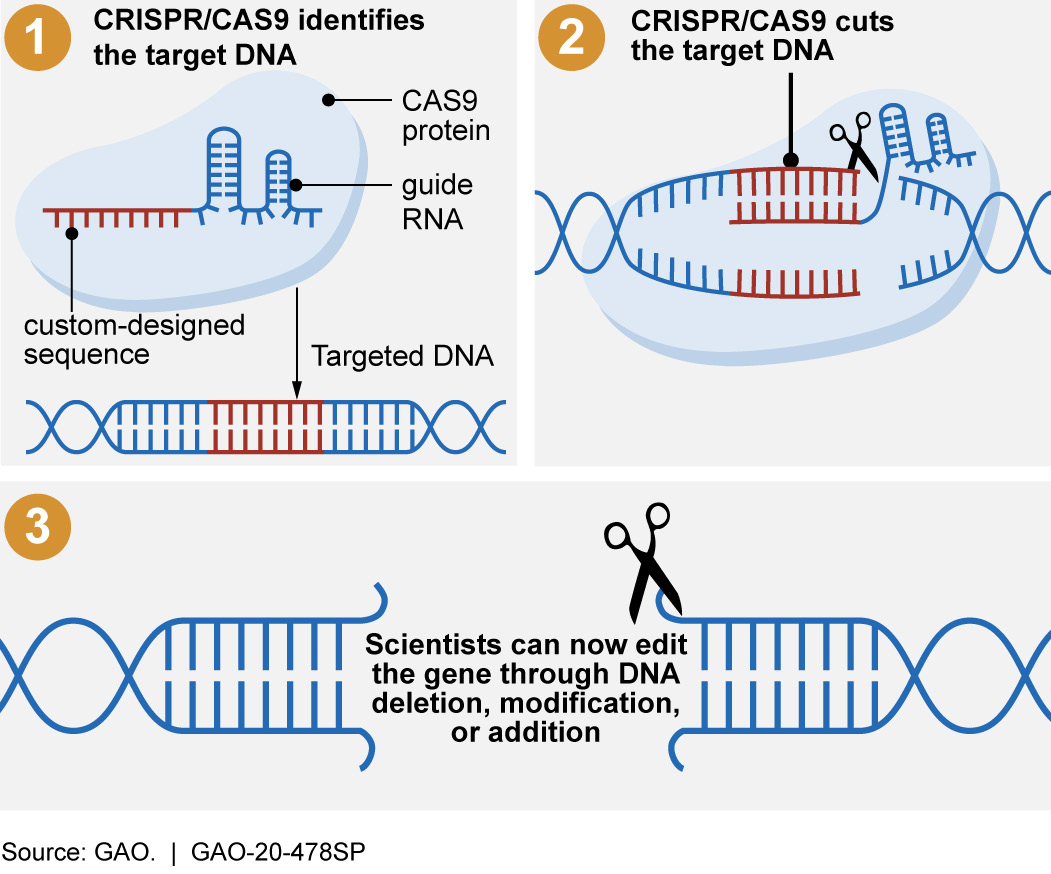
Figure 1. CRISPR/CAS9 systems allow scientists to make targeted changes to an organism’s DNA.
Researchers are already building on CRISPR to create new gene editing systems. For example, a system known as prime editing builds on the knowledge of CRISPR/CAS9 to directly exchange one piece of the DNA with another, which is known as base editing.
How mature is it?
Since its development in 2012, CRISPR has generated significant excitement because it is faster, cheaper, more accurate, and more efficient than prior methods. As a result, interest has grown rapidly, and CRISPR is now used extensively in thousands of laboratories worldwide.
Further, CRISPR-based treatments for certain diseases, including sickle cell anemia and some types of cancer, are currently in clinical trials. However, CRISPR has not yet been approved by the U.S. Food and Drug Administration (FDA) for medical uses, nor has FDA issued regulations on food safety for crops edited with CRISPR.
Opportunities
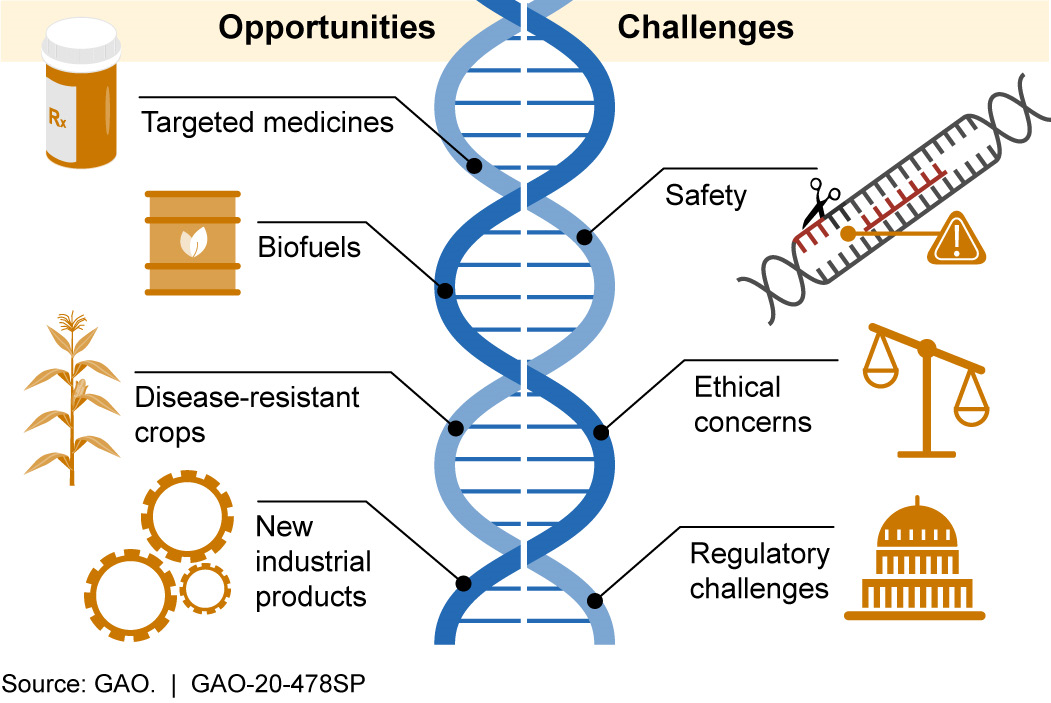
Figure 2. Examples of CRISPR’s range of possible opportunities and challenges.
CRISPR has the potential to help create more advanced products in many sectors that have a major impact on society. Examples include:
- Development of new diagnostic tests, targeted medicines, and treatments. By pairing CRISPR with other CAS proteins that cut the target DNA or RNA and then cut other molecules to produce a visual signal, scientists can identify when viral components are present. Scientists are using this technology to develop diagnostic tests that may rapidly identify diseases such as COVID-19. CRISPR/ CAS9 is being tested for possible treatment for certain diseases, including sickle cell anemia and some types of cancer. CRISPR may also be used to help control certain diseases by altering the traits of insects or other organisms that can transmit disease. For example, scientists used CRISPR to make mosquitoes more resistant to the parasite that causes malaria.
- Stronger, more disease-resistant crops. CRISPR may improve agricultural products by increasing nutrient content, disease resistance, and survivability in adverse weather and soil conditions. For example, when researchers deleted a small portion of a specific cucumber gene, the plants were less susceptible to viruses known to damage the plants and cause significant crop losses.
- More advanced industrial products. Various industries are exploring the potential use of CRISPR to develop new biofuels, bacteria that could clean up environmental disasters, and new materials.
Challenges
CRISPR gene editing has also raised concerns in areas such as safety, ethics, and regulation. Examples include:
- Safety concerns. There could be unintended consequences of using CRISPR. For example, it could target an unintended location within the DNA, producing changes that could cause disease or other harm. While some research indicates errors may be rare, researchers do not fully understand the consequences of such errors. Additionally, CRISPR activity may stress cells and prevent editing. Further, while some cells can recover after their DNA has been altered, others cannot.
- Ethical concerns. Ethical concerns include whether CRISPR would be used for enhanced human characteristics, such as increased muscle mass, learning aptitude, and memory, and if there would be equitable access to all populations. The potential to use CRISPR to edit the human germline (eggs, sperm, and very early embryos) also raises ethical questions. For example, to what extent might genetic changes affect future generations, and should research on human embryos be allowed? In March 2018, a group of 18 scientists from seven countries, including the U.S., Canada, France, and China, called for a voluntary moratorium on all studies involving gene editing of the human germline.
- Regulatory challenges. Many countries are struggling with questions of how to regulate CRISPR and other gene editing technologies. For example, in the United States, it is currently unclear whether gene edited crops will be subject to the same regulations as conventional genetically modified (GM) organisms. In 2017, FDA began seeking public input on its regulatory policy, but, as of March 2020, it has not published new guidance for industry on its current regulatory policy. In Europe, a 2018 court ruling determined that gene edited crops would be subject to the same regulations as GM plants.
Policy Context and Questions
- How should regulations address the use of CRISPR and related gene editing technologies? How should regulations be tailored to applications that affect animals, plants, and humans?
- What federal agencies should be responsible for determining the safety of commercial products developed with CRISPR and related gene editing technologies?
- What are the legal, societal, and environmental implications of using CRISPR and related gene editing technologies? How should the federal government address those implications?
For more information, contact Karen Howard at (202) 512-6888 or HowardK@gao.gov.
