New Efforts to Fight Food Poisoning
News of a food recall can cause you to race to the refrigerator to check that the lettuce you just bought wasn’t part of the recall.
While recalls can raise concerns, they are also a good thing, as they are valuable reminders of the government’s work to keep the U.S. food supply safe. The ability to quickly trace tainted foods to the source of contamination and recall a potentially contaminated product can help prevent illnesses and improve public health and safety.
Some foods—such as fresh produce and eggs—are more frequently associated with foodborne illnesses than others. In November 2022, the Food and Drug Administration (FDA) began taking steps to change how it traces certain foods through the supply chain.
Today’s WatchBlog post looks at our new report on FDA’s efforts and some of the challenges facing the agency.
Image
Costly outbreaks of foodborne illnesses
FDA and industry officials recognize that outbreaks of foodborne illnesses have both health and other costs. For example, from 2014 through 2021, outbreaks linked to leafy greens were associated with a total of 2,028 reported illnesses, 477 hospitalizations, and 18 deaths, according to the Centers for Disease Control and Prevention.
Outbreaks can also be costly because of product losses from overly broad recalls. For example, in November 2018, FDA issued a public advisory about a multi-state outbreak linked to romaine lettuce. It advised against eating any romaine lettuce on the market at that time, as it didn’t have enough information to identify the source of contamination that would allow a more targeted recall. As a result, total losses from the recall—including damages to consumers and suppliers—was estimated to be between $320 to $400 million.
In addition to these financial impacts, recurring outbreaks and recalls can reduce the public’s trust in the food supply.
What is FDA doing to improve tracing?
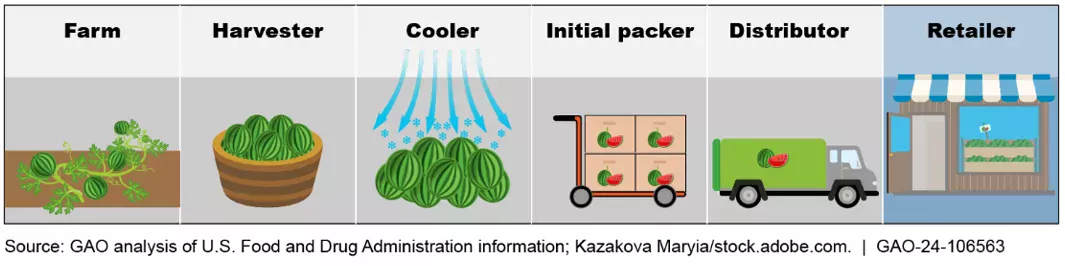
The FDA Food Safety Modernization Act directed FDA to establish additional recordkeeping requirements for entities—such as farmers, distributors, or retailers—that handle foods FDA has designated as high risk to public health. The date for these entities to comply with the enhanced recordkeeping requirements is January 20, 2026. Once they are in place, they will allow FDA and industry to identify the source of foodborne illness more quickly, issue targeted recalls, and limit public exposure to products of concern.
FDA has developed a list of foods for which additional traceability records are required, such as items that have historically been involved in outbreaks. Examples of products on this list are nut butters, some fresh produce, and ready-to-eat deli salads.
When carrying out the recordkeeping requirements, entities handling foods on the list will have to, among other things, maintain specific records at certain points in the food’s supply chain. They must also maintain a traceability plan and provide FDA with specific traceability information within 24 hours of a request.
What should FDA do to improve its efforts?
FDA has set target dates for implementing and enforcing its new recordkeeping requirements. But it hasn’t finalized an implementation plan. These plans should detail, among other things, FDA’s efforts to work with nonfederal regulators to enforce the new requirements. They should also include outreach and education plans for the industry that will help them comply with the new requirements.
Groups representing nonfederal regulators and industry stakeholders told us that without this detailed information, it’ll be hard for them to effectively enforce or comply with the requirements. As a result, we recommended that FDA finalize and document an implementation plan to help the agency meet its regulatory goals.
Learn more about FDA’s efforts to improve food safety by checking out our new report.
- GAO’s fact-based, nonpartisan information helps Congress and federal agencies improve government. The WatchBlog lets us contextualize GAO’s work a little more for the public. Check out more of our posts at GAO.gov/blog.
GAO Contacts
Related Products

GAO's mission is to provide Congress with fact-based, nonpartisan information that can help improve federal government performance and ensure accountability for the benefit of the American people. GAO launched its WatchBlog in January, 2014, as part of its continuing effort to reach its audiences—Congress and the American people—where they are currently looking for information.
The blog format allows GAO to provide a little more context about its work than it can offer on its other social media platforms. Posts will tie GAO work to current events and the news; show how GAO’s work is affecting agencies or legislation; highlight reports, testimonies, and issue areas where GAO does work; and provide information about GAO itself, among other things.
Please send any feedback on GAO's WatchBlog to blog@gao.gov.




