Could AI Help Create New Medicines?
Developing and bringing a new drug to market is a lengthy and expensive process. Only about 1 in 10,000 chemical compounds that are tested makes it through the research and development pipeline and is approved by the FDA.
This process can take 10 to 15 years (and cost between $600 million and $1.4 billion) for a single drug.
We looked at how machine learning—a form of artificial intelligence in which software uses huge amounts of data to independently perform a task—could help speed up this process and make it cheaper.
Read on, and listen to our podcast with GAO’s Chief Scientist and Managing Director of Science, Technology Assessment and Analytics, to learn more.
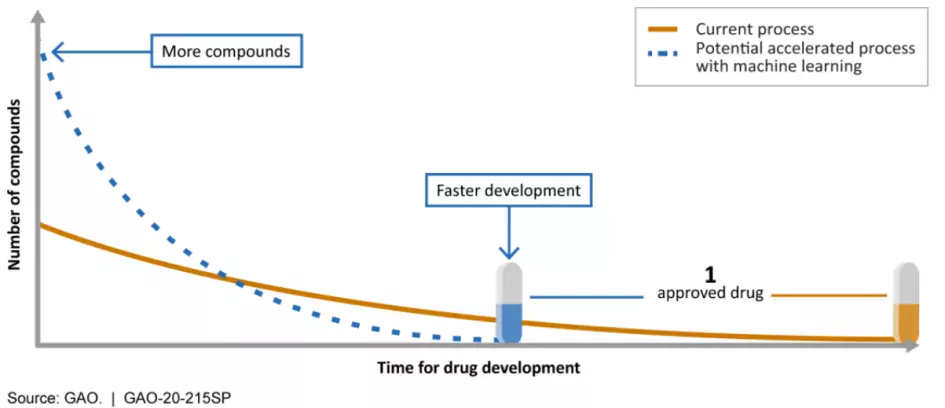
AI could improve discovery and testing…
Machine learning is already being used throughout the drug development process.
- Drug discovery: Machine learning is helping researchers identify and screen more chemical compounds, and screen existing compounds for new therapeutic applications.
- Preclinical research: Researchers are using machine learning to augment preclinical testing and predict toxicity before testing potential drugs on humans. This could help weed out compounds that will fail in clinical trials, which could reduce costs.
- Clinical trials: Machine learning is helping improve clinical trial design (a point where many drug candidates fail) by assisting in patient selection and recruitment.
Machine learning might screen more compounds and help develop drugs faster
These improvements could save lives and reduce suffering by getting drugs to patients more quickly. This could also allow researchers to invest more resources in areas like rare or orphan diseases.
But challenges need to be addressed
Before patients see these benefits, however, researchers and drug companies will have to wrestle with a number of challenges. For example, a shortage of high-quality data is a major challenge for machine learning in drug development. Accessing and sharing data can also be difficult due to cost, legal issues, and reluctance to share data from some companies.
Additionally, a shortage of skilled workers makes hiring and retention difficult for drug companies and regulators. Gaps in research and uncertainty about federal regulations may also hurt the adoption of this technology.
We’ve identified a number of policy options that the federal government and other leaders could use to address these issues. These include creating incentives to increase data sharing, and developing a clear and consistent message regarding regulation.
Find out more by reading the full report.
This report is part of a larger collaboration between GAO and the National Academy of Medicine and includes an excerpt from the Academy’s 2020 special publication, Artificial Intelligence in Health Care: The Hope, the Hype, the Promise, the Peril.
- Comments on GAO’s WatchBlog? Contact blog@gao.gov.
GAO Contacts
Related Products

GAO's mission is to provide Congress with fact-based, nonpartisan information that can help improve federal government performance and ensure accountability for the benefit of the American people. GAO launched its WatchBlog in January, 2014, as part of its continuing effort to reach its audiences—Congress and the American people—where they are currently looking for information.
The blog format allows GAO to provide a little more context about its work than it can offer on its other social media platforms. Posts will tie GAO work to current events and the news; show how GAO’s work is affecting agencies or legislation; highlight reports, testimonies, and issue areas where GAO does work; and provide information about GAO itself, among other things.
Please send any feedback on GAO's WatchBlog to blog@gao.gov.